137,99 €
Mehr erfahren.
- Herausgeber: John Wiley & Sons
- Kategorie: Wissenschaft und neue Technologien
- Serie: Wiley Series in Protein and Peptide Science
- Sprache: Englisch
The first and only book devoted entirely to MEMBRANE LIPID ASYMMETRY AND ITS BIOLOGICAL IMPLICATIONS
Transmembrane Dynamics of Lipids is comprised of contributions from expert authors from leading research groups that present up-to-date quantitative data on the formation, stability, and biological consequences of the asymmetrical organization of lipids in cell membranes.
Incorporating an impressive amount of new, previously uncollected data, the book examines transmembrane asymmetry and movement of lipids in biological membranes, and methods for the measurement of transmembrane lipid motion, emphasizing the role of lipid flippases and discusses biological functions associated with lipid asymmetry. In addition, it draws attention to important new discoveries in the field, such as the correlation between malfunction of lipid flippases and human diseases such as thrombosis and cancer. The book also addresses the manifold methods that are used to measure the rate of transmembrane movement of lipids in biological and model systems.
The only guide to new discoveries regarding lipids in cell membranes, Transmembrane Dynamics of Lipids is designed to appeal to biophysicists, biochemists, and cellular and molecular biologists working in the growing field of membrane research.
Sie lesen das E-Book in den Legimi-Apps auf:
Seitenzahl: 771
Veröffentlichungsjahr: 2011
Ähnliche
Table of Contents
Cover
WILEY SERIES ON PROTEIN AND PEPTIDE SCIENCE
Title page
Copyright page
INTRODUCTION
HISTORICAL PERSPECTIVES: WHO DID WHAT AND WHAT’S NEXT?
BIOLOGICAL ADVANTAGES OF LIPID ASYMMETRY
PROSPECTS
ORGANIZATION OF THIS BOOK
ACKNOWLEDGMENTS
LIST OF CONTRIBUTORS
PART I: ASSESSING TRANSMEMBRANE MOVEMENT AND ASYMMETRY OF LIPIDS
1 METHODS FOR THE DETERMINATION OF LIPID TRANSMEMBRANE DISTRIBUTION AND MOVEMENT IN BIOLOGICAL MEMBRANES
1.1 INTRODUCTION
1.2 DEVELOPMENT OF ASSAYS FOR DISTRIBUTION AND TRANSLOCATION OF LIPIDS ACROSS MEMBRANES
1.3 OVERVIEW ON ASSAYS FOR MEASURING DISTRIBUTION AND TRANSLOCATION OF LIPIDS ACROSS CELLULAR MEMBRANES
1.4 MAIN TECHNIQUES USED TO DETERMINE TRANSBILAYER DISTRIBUTION OF ENDOGENOUS LIPIDS IN CELL MEMBRANES
1.5 MAIN TECHNIQUES USED TO DETERMINE TRANSBILAYER DISTRIBUTION OF LIPID ANALOGS IN CELL MEMBRANES
2 DETECTION AND MEASUREMENT OF UNLABELED LIPID TRANSMEMBRANE MOVEMENT
2.1 INTRODUCTION
2.2 MEASUREMENT OF TRANSMEMBRANE FLIP-FLOP OF UNLABELED LIPIDS BY SHAPE CHANGE OF GUVs
2.3 MEASUREMENT OF TRANSMEMBRANE FLIP-FLOP OF UNLABELED LIPIDS USING AFM
2.4 CONCLUSIONS
ACKNOWLEDGMENTS
PART II: LIPID ASYMMETRY IN CELL MEMBRANES
3 NEW INSIGHTS IN MEMBRANE LIPID ASYMMETRY IN ANIMAL AND PLANT CELLS
3.1 LIPID ASYMMETRY IN ANIMAL MEMBRANES
3.2 CREATING, MAINTAINING, OR RANDOMIZING THE MEMBRANE PHOSPHOLIPID DISTRIBUTION: PHOSPHOLIPID TRANSPORTERS
3.3 WHAT ABOUT LIPID ASYMMETRY AND TRANSLOCATION IN PLANT CELL MEMBRANES?
4 SPHINGOLIPID ASYMMETRY AND TRANSMEMBRANE TRANSLOCATION IN MAMMALIAN CELLS
4.1 INTRODUCTION
4.2 SPHINGOSINE, SPHINGOSINE-1-PHOSPHATE, AND CERAMIDE
4.3 CERAMIDE
4.4 GLYCOSPHINGOLIPIDS
4.5 SPHINGOMYELIN
4.6 FUTURE PERSPECTIVES
5 TRANSBILAYER MOVEMENT AND DISTRIBUTION OF CHOLESTEROL
5.1 INTRODUCTION
5.2 PHYSICOCHEMICAL FEATURES OF CHOLESTEROL
5.3 METHODS FOR MEASURING CHOLESTEROL TRANSBILAYER MOVEMENT AND DISTRIBUTION
5.4 TRANSBILAYER MOVEMENT OF CHOLESTEROL IN MODEL MEMBRANES
5.5 TRANSBILAYER MOVEMENT OF CHOLESTEROL IN BIOLOGICAL MEMBRANES
5.6 TRANSBILAYER DISTRIBUTION OF CHOLESTEROL IN LIPID AND BIOLOGICAL MEMBRANES
5.7 CHOLESTEROL FLIP-FLOP: FAST OR SLOW?
5.8 ROLE OF PROTEINS IN THE TRANSPORT OF CHOLESTEROL ACROSS MEMBRANES
5.9 CONCLUDING REMARKS
ACKNOWLEDGMENT
PART III: ENERGY-INDEPENDENT PROTEIN-MEDIATED TRANSMEMBRANE MOVEMENT OF LIPIDS
6 PHOSPHOLIPID FLIP-FLOP IN BIOGENIC MEMBRANES
6.1 INTRODUCTION
6.2 ASSAYS FOR MEASURING TRANSBILAYER DISTRIBUTION OF ENDOGENOUS PHOSPHOLIPIDS
6.3 ASSAYS FOR MEASURING TRANSBILAYER DISTRIBUTION AND MOVEMENT OF PHOSPHOLIPID ANALOGS
6.4 SHAPE CHANGES OF GUVs AS A TOOL TO MEASURE FLIP-FLOP
6.5 TRANSBILAYER MOVEMENT OF PHOSPHOLIPIDS IN THE ER
6.6 TRANSBILAYER MOVEMENT OF PHOSPHOLIPIDS IN THE BACTERIAL INNER MEMBRANE
6.7 MECHANISM OF RAPID LIPID FLIP-FLOP IN BIOGENIC MEMBRANES
6.8 EFFORTS TO IDENTIFY PHOSPHOLIPID FLIPPASES
6.9 FLIPPING OF ISOPRENOID-BASED GLYCOLIPIDS
6.10 CONCLUSION
7 PHOSPHOLIPID SCRAMBLASE: WHEN PHOSPHOLIPID ASYMMETRY GOES AWAY
7.1 INTRODUCTION
7.2 HISTORICAL OVERVIEW
7.3 PHYSIOLOGICAL IMPORTANCE OF LIPID SCRAMBLING
7.4 CHARACTERISTICS OF THE PHOSPHOLIPID SCRAMBLING PROCESS
7.5 TOWARD IDENTIFICATION: PROPOSED CANDIDATE PROTEINS AND MECHANISMS
7.6 CONCLUDING REMARKS
PART IV: ENERGY-DEPENDENT LIPID TRANSPORT ACROSS MEMBRANES
8 FLIP OR FLOP: MECHANISM AND (PATHO)PHYSIOLOGY OF P4-ATPASE-CATALYZED LIPID TRANSPORT
8.1 INTRODUCTION
8.2 P4-ATPASES ARE PRIME CANDIDATE PHOSPHOLIPID TRANSLOCASES
8.3 MECHANISM OF P4-ATPASE-CATALYZED LIPID TRANSPORT: ROLE OF ACCESSORY SUBUNITS
8.4 ROLE OF P4-ATPASES IN VESICLE-MEDIATED PROTEIN TRANSPORT
8.5 P4-ATPASE DYSFUNCTION AND DISEASE
8.6 FUTURE CHALLENGES
ACKNOWLEDGMENTS
9 COUPLING DRS2P TO PHOSPHOLIPID TRANSLOCATION, MEMBRANE ASYMMETRY, AND VESICLE BUDDING
9.1 INTRODUCTION
9.2 P4-ATPASES IN BUDDING YEAST
9.3 EVIDENCE THAT DRS2P IS A FLIPPASE
9.4 DRS2P IN PROTEIN TRANSPORT AND VESICLE BUDDING
9.5 CONCLUDING REMARKS
10 SUBSTRATE SPECIFICITY OF THE AMINOPHOSPHOLIPID FLIPPASE
10.1 INTRODUCTION
10.2 SUBSTRATE SPECIFICITY OF THE PM AMINOPHOSPHOLIPID FLIPPASE
10.3 IDENTIFICATION AND SUBSTRATE SPECIFICITY OF CANDIDATE AMINOPHOSPHOLIPID FLIPPASES
10.4 IS THE LIPID SPECIFICITY OF CANDIDATE AMINOPHOSPHOLIPID FLIPPASES UNIQUE?
10.5 LIPID SPECIFICITY OF OTHER PS-BINDING PROTEINS
10.6 SEQUENCE ELEMENTS THAT BIND TO PS
10.7 CONCLUSIONS
ACKNOWLEDGMENTS
11 THE FLIPPASE DELUSION?
11.1 ATP-BINDING CASSETTE (ABC) TRANSPORTERS AND LIPID FLIP-FLOP
11.2 ABCA4 AND LIPID TRANSLOCATION: EXPLAINING A PHENOTYPE?
11.3 MsbA AND LIPID TRANSLOCATION: A KEY TO SURVIVAL
11.4 DRUG AND LIPID MOVEMENT BY ABCB1: IS THE MECHANISM A FLIP-FLOP?
11.5 ABCB4: THE FORGOTTEN AND LIKELY LIPID FLIPPASE?
11.6 CONCLUSIONS AND PERSPECTIVES
PART V: RELEVANCE OF LIPID TRANSMEMBRANE DISTRIBUTION FOR MEMBRANE PROPERTIES AND PROCESSES
12 MEMBRANE LIPID ASYMMETRY AND PERMEABILITY TO DRUGS: A MATTER OF SIZE
12.1 INTRODUCTION
12.2 THE ORIGIN OF LIPINSKI’S SECOND RULE FROM THE POINT OF VIEW OF THE PHARMACEUTICAL INDUSTRY
12.3 SOLVING LIPINSKI’S SECOND RULE
12.4 LIPINSKI’S SECOND LAW AND POTENTIAL APPLICATION
12.5 CONCLUSION
ACKNOWLEDGMENT
13 ENDOCYTOSIS AND LIPID ASYMMETRY
13.1 INTRODUCTION
13.2 BENDING A MEMBRANE
13.3 SHAPE CHANGES OF GUVs INDUCED BY LIPID ASYMMETRY
13.4 HOW ENDOCYTOSIS IS LINKED TO LIPID ASYMMETRY
13.5 ROLE OF P4-ATPases IN THE FORMATION OF ENDOCYTIC INVAGINATIONS
13.6 CONCLUDING REMARKS
ACKNOWLEDGMENTS
PART VI: APOPTOSIS AND DISEASES: CONSEQUENCES OF DISRUPTION TO LIPID TRANSMEMBRANE ASYMMETRY
14 MEMBRANE LIPID ASYMMETRY IN AGING AND APOPTOSIS
14.1 INTRODUCTION
14.2 PHOSPHOLIPID TRANSPORTERS
14.3 LIPID ASYMMETRY IN ERYTHROCYTES
14.4 LIPID ASYMMETRY DURING APOPTOSIS
14.5 CA2+ HOMEOSTASIS DURING APOPTOSIS
14.6 MEMBRANE PHOSPHOLIPID ASYMMETRY: STATIC OR DYNAMIC?
14.7 REGULATION OF LIPID ASYMMETRY DURING APOPTOSIS
14.8 SIGNIFICANCE
14.9 CONCLUDING REMARKS
15 PHOSPHATIDYLSERINE EXPOSURE IN HEMOGLOBINOPATHIES
15.1 INTRODUCTION
15.2 RBC PHOSPHOLIPID ORGANIZATION
15.3 THE RBC FLIPPASE
15.4 PS EXPOSURE IN RBCS
15.5 PS EXPOSURE IN HEMOGLOBINOPATHIES
15.6 CONSEQUENCES OF PS EXPOSURE
15.7 PHOSPHOLIPID TRANSBILAYER MOVEMENT IN HEMOGLOBINOPATHIES
15.8 CONCLUSION
16 SCOTT SYNDROME: MORE THAN A HEREDITARY DEFECT OF PLASMA MEMBRANE REMODELING
16.1 INTRODUCTION
16.2 SCOTT SYNDROME FEATURES AND PHENOTYPE
16.3 CELL BIOLOGY OF SCOTT SYNDROME
16.4 CANDIDATE PROTEINS IN THE TRANSMEMBRANE REDISTRIBUTION OF PS
16.5 THE SIGNIFICANCE OF MEMBRANE VESICULATION AND OF DERIVED MPs
16.6 WHAT CAN BE LEARNED FROM SCOTT SYNDROME?
16.7 CONCLUSION
17 ABCA1, TANGIER DISEASE, AND LIPID FLOPPING
17.1 HISTORICAL NOTES: TANGIER DISEASE (TD) AND ATP-BINDING CASSETTE TRANSPORTER 1 (ABCA1)
17.2 THE ABCA1 GENE AND THE REGULATION OF ITS EXPRESSION
17.3 THE ABCA1 PROTEIN AND ITS INTERACTIONS
17.4 ABCA1: MUTATIONS AND CLINICAL SIGNS
17.5 TARGETED INACTIVATION AND OVEREXPRESSION OF ABCA1 IN ANIMAL MODELS
17.6 LIVER AND MACROPHAGE ABCA1: LIPID EFFLUX AND HDL FORMATION
17.7 ABCA1 AND MEMBRANE FUNCTION
17.8 ABCA1: LIPID FLOP AND LIPID EFFLUX
17.9 ABCA1 AND THE LIPID MICROENVIRONMENT AT THE MEMBRANE
17.10 CONCLUSIONS
ACKNOWLEDGMENTS
Index
WILEY SERIES ON PROTEIN AND PEPTIDE SCIENCE
VLADIMIR N. UVERSKY, Series Editor
Metalloproteomics• Eugene A. Permyakov
Instrumental Analysis of Intrinsically Disordered Proteins: Assessing Structure and Conformation• Vladimir Uversky, Sonia Longhi
Protein Misfolding Diseases: Current and Emerging Principles and Therapies• Marina Ramirez-Alvarado, Jeffery W. Kelly, Christopher M. Dobson
Calcium Binding Proteins• Eugene A. Permyakov and Robert H. Kretsinger
Protein Chaperones and Protection from Neurodegenerative Diseases• Stephan Witt
Transmembrane Dynamics of Lipids• Philippe Devaux and Andreas Herrmann
Introduction to the Wiley Series on Protein and Peptide Science
Proteins and peptides are the major functional components of the living cell. They are involved in all aspects of the maintenance of life. Their structural and functional repertoires are endless. They may act alone or in conjunction with other proteins, peptides, nucleic acids, membranes, small molecules, and ions during various stages of life. Dysfunction of proteins and peptides may result in the development of various pathological conditions and diseases. Therefore, the protein/peptide structure–function relationship is a key scientific problem lying at the junction point of modern biochemistry, biophysics, genetics, physiology, molecular and cellular biology, proteomics, and medicine.
The Wiley Series on Protein and Peptide Science is designed to supply a complementary perspective from current publications by focusing each volume on a specific protein- or peptide-associated question and endowing it with the broadest possible context and outlook. The volumes in this series should be considered required reading for biochemists, biophysicists, molecular biologists, geneticists, cell biologists, and physiologists, as well as those specialists in drug design and development, proteomics, and molecular medicine with an interest in proteins and peptides. I hope that each reader will find in the volumes within this book series interesting and useful information.
First and foremost, I would like to acknowledge the assistance of Anita Lekhwani of John Wiley & Sons, Inc., throughout this project. She has guided me through countless difficulties in the preparation of this book series, and her enthusiasm, input, suggestions, and efforts were indispensable in bringing the Wiley Series on Protein and Peptide Science into existence. I would like to take this opportunity to thank everybody whose contribution in one way or another has helped and supported this project. Finally, special thank you goes to my wife, sons, and mother for their constant support, invaluable assistance, and continuous encouragement.
Vladimir N. Uversky
September 2008
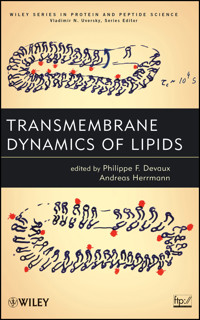
The cover shows a transparency used by P.D. in presentations given in the days before PowerPoint was available. The cartoons illustrate the principal of the ascorbate assay to assess the transbilayer motion and distribution of spin-labeled lipids in membranes taking the plasma membrane of red blood cells as an example (see Preface and Chapters 1 and 6). Lower cartoon: Spin-labeled lipids (red) were incorporated into the outer leaflet of the plasma membrane and redistributed between both leaflets. Upper cartoon: Ascorbate was added to the cell suspension reducing spin-labeled lipids selectively on the outer leaflet. By comparing the signal intensity of spin-labeled lipids without and with ascorbate, the transbilayer distribution of labeled lipid analogs can be measured. The transparency has been used many times (see the spread colors on the left). Just by chance, it was rediscovered among an amazing pile of reprints in the office of P.D.
Copyright © 2012 by John Wiley & Sons, Inc. All rights reserved
Published by John Wiley & Sons, Inc., Hoboken, New Jersey
Published simultaneously in Canada
No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 750-4470, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley.com/go/permissions.
Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives or written sales materials. The advice and strategies contained herein may not be suitable for your situation. You should consult with a professional where appropriate. Neither the publisher nor author shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages.
For general information on our other products and services or for technical support, please contact our Customer Care Department within the United States at (800) 762-2974, outside the United States at (317) 572-3993 or fax (317) 572-4002.
Wiley also publishes its books in a variety of electronic formats. Some content that appears in print may not be available in electronic formats. For more information about Wiley products, visit our web site at www.wiley.com.
Library of Congress Cataloging-in-Publication Data:
Transmembrane dynamic lipids / edited by Philippe Devaux, Andreas Herrmann.
p. cm. – (The Wiley series in protein and peptide science ; 9)
Includes index.
ISBN 978-0-470-38845-7 (hardback)
1. Membrane lipids. 2. Cell membranes. 3. Membrane transport. I. Devaux, Philippe F. II. Herrmann, Andreas.
QP752.M45T73 2012
572'.577–dc23
2011021424
eISBN: 9781118120088
ePub: 9781118120101
oISBN: 9781118120118
MOBI: 9781118120095
INTRODUCTION
HISTORICAL PERSPECTIVES: WHO DID WHAT AND WHAT’S NEXT?
Ole Mouritsen, in his recent monograph entitled “Lipids—As a Matter of Fat,” summarized with humor the views of many biologists concerning lipids, as follows: “Lipids appear to play a fairly non-specific role, being rather dull and anonymous compared to fashionable stuff like the proteins that catalyze all biochemical reactions and the genes that contain the information needed to produce proteins” [1].
The present book, which is addressed to researchers, teachers, and students in cell biology and in biochemistry, has the goal of convincing all scientists that lipids, on the contrary, have sophisticated behaviors and play multiple important roles in living organisms. It is also addressed to physicists fascinated by the various spontaneous self-organization of lipids in water (lipid polymorphism) to warn them that lipids in biological systems are not always at thermal equilibrium, and that phase separations and lateral or transmembrane domains seen in model systems can differ fundamentally from biological situations. Indeed, molecule segregation in biological systems results often from the work of ATPases, like the flippases, or is the result of a molecule sorting by “protein gates” (see the “fence and picket model” of Kusumi and collaborators [2]). Such mechanisms are difficult to mimic in model systems.
In any case, all lipids are not equivalent and their chemical heterogeneity, for example, between the two sides of a biomembrane, is the result of a long selection during evolution, which allows lipids to fulfill different functions, from that of a fluid hydrophobic medium for membrane proteins to that of selective messenger molecules and enzyme cofactors. In the latter case, they have to find their partners in a cell, hence to move rapidly in a very anisotropic environment.
To many biologists, lipids form the third class of molecules of living organism after proteins and nucleic acids. Yet, lipids were probably not the third in the evolution nor are they third in importance, since a cell and even many viruses cannot exist without a membrane. The fact is that lipids form the building blocks of biological membranes. They determine the boundary of all living organisms as well as the compartmentalization of organelles in eukaryotes. Regarded as passive molecules forming only viscous cement that holds membrane proteins, filtering out hydrophilic molecules, the lipid bilayer is in reality a sophisticated structure capable of a remarkable polymorphism in water. The physical characteristic of a lipid bilayer permits not only protein movement but also membrane deformations and, coupled to the cytoskeleton, provides the cell membrane with mechanical properties. Not the least astonishing is the bilayer’s ability to divide in two compartments during cell division without losing molecules in the plasma due to efficient self-sealing capacities. Nonetheless, there are still mysteries concerning lipids, which are matters of research, speculation, and controversy. (1) Biophysicists have succeeded in making stable membranes (liposomes) with only one type of lipids, in suspension in water, for example, with egg phosphatidylcholine (PC), while biological membranes harbor several hundred different lipids. Why are there so many chemically different lipids coexisting in nature? (2) Why is the lipid composition of various membranes of eukaryotes different and sometimes even the two sides of biological membranes different (asymmetrical)? This requires numerous specific enzymes for the synthesis and ultimately for the shuttling to the right destination of newly formed lipids. Is such a multiplicity necessary for a fine-tuning of membrane-bound enzymes or is the variety of lipids used to give specific messages to specific proteins? Is the detailed chemical structure of lipids without real importance and does it reflect only the precursor molecules available? Not only do eukaryotic membranes have many chemically different lipids if one considers chain length, unsaturation, and polar head group, but also the lipids are not homogenously distributed within the various organelles and even between the different sides of one membrane. This lipid heterogeneity, a “complication of Nature,” was transmitted more than a million years in eukaryotic cells and has survived the filter of evolution, suggesting that the lipid composition and distribution within a cell is neither accidental nor inconsequential for the activity of cells.
Although cells tolerate certain variability in lipid composition, many human diseases have been associated with the inability of mutated cells to synthesize specific lipids or to recycle particular lipids from the nutriments or to address specific lipids to their correct destination. Alternatively, the excess of certain lipids such as cholesterol or saturated phospholipid chains can be poisonous.
In the late 1960s, V. Luzzati, in a pioneer work carried out in France, showed by X-ray crystallography that lipids extracted from biological membranes form, in water, lamellar phases, giving rise spontaneously to large multilamellar (onion-style) liposomes made of a superposition of bilayers [3, 4]. Physicists characterized the bilayers as liquid crystals that could be in a fluid state or in a more viscous, gel state. In the early 1970s, the concept of lipid bilayer emerged as the basic model of biomembranes and was popularized in the famous model of “fluid mosaic membrane” of S.J. Singer and G.L. Nicolson [5]. Although the concept of “mosaicity” implies the presence of heterogeneous lateral domains, and in spite of the work carried out by several physical chemists such as H. McConnell, it was only in 1997 (almost 30 years after the initial work of Luzzati and McConnell) that the importance of lateral domains began to be popular among membranologists and that biological functions associated with lateral domains (or rafts) were highlighted (see the work of K. Simons and E. Ikonen [6]).
Indeed, the two monolayers of biomembranes form distinct lipid domains: M. Bretscher in England demonstrated in the early 1970s the asymmetrical transmembrane distribution of phospholipids in the plasma membrane of human erythrocytes [7]. Bretscher used the chemical labeling of the amino groups of phosphatidylserine (PS) or phosphatidylethanolamine (PE) and showed that aminophospholipids are principally in the membrane inner monolayer, while PC and sphingomyelin (SM) are essentially in the outer monolayer of human red cells. Subsequent investigation in the laboratory of L.L.M. van Deenen in The Netherlands based on phospholipases and sphingomylinases assays [8, 9] confirmed Bretscher’s results and demonstrated that the transmembrane asymmetry of red cells is an ubiquitous property of the plasma membrane of eukaryotes. In model systems, on the other hand, no transmembrane lipid segregation was found to form spontaneously. Sonication allows one to achieve a lipid sorting between inner and outer monolayers in small unilamellar vesicles (SUVs), but the latter structures are not physiological because of their small size compared with that of vesicles produced in vivo (∼20-nm diameter for SUVs vs. ∼200 nm for endocytic vesicles). Thus, lipid sorting observed in biomembranes had to be caused by a process that does not exist in liposomes and is not a mere thermodynamic equilibrium. Initially, the segregation of aminophospholipids was believed to be due to the topology of enzymes responsible for lipid synthesis or to lipid–cytoskeleton interactions (J.A.F. Op den Kamp [10]). However, Bretscher had the remarkable intuition to postulate the existence of specific lipid enzymes that he named “phospholipid flippase,” which would be responsible for the establishment of the asymmetrical lipid organization at the expense of ATP hydrolysis. In practice, it was later found necessary to specify the orientation of the postulated lipid carrier and the requirement or absence of requirement for ATP hydrolysis. This explains why the habit is now to differentiate among flippase, floppase, and scramblase (Fig. I.1).
Figure I.1. Definition of the various lipid transporters in eukaryotic cell membranes. Note that the scramblase is calcium dependent and that “flippase” is a term that is used sometimes to designate an enzyme that catalyzes lipid flip-flop in both directions (inward or outward), for example, in the endoplasmic reticulum.
A prerequisite for stable lipid segregation between the two monolayers of a membrane is a priori a slow transmembrane diffusion. In 1971, R.D. Kornberg and H.M. McConnell at Stanford University demonstrated for the first time, with spin-labeled lipids, the very slow transmembrane diffusion of phospholipids in sonicated lipid vesicles, where the “flip-flop” between the two monolayers was found to require several hours at 30°C [11]. It is now admitted that the spontaneous transmembrane diffusion of lipids is very slow in liposomes of any size as well as in biological membranes. A few exceptions to this rule were discovered recently. Cholesterol, ceramide, phospatidic acid, diacylglycerol, and free fatty acids or esters have a rapid spontaneous diffusion (τ1/2 less than 1 minute). The absence of real polar head groups in such lipids probably explains this unusual result.
It was only in 1984, that is, more than 10 years after Bretscher’s hypothesis, that the existence of a phospholipid flippase was demonstrated in France by M. Seigneuret and P.F. Devaux in the human erythrocyte membrane using spin-labeled analogs of naturally occurring phospholipids [12] and the year after by D.L. Daleke and W.H. Huestis, who provided confirmation using an elegant technique involving nonlabeled lipids [13], while A. Schroit’s group [14] took advantage of fluorescent analogs to prove the existence of an erythrocyte aminophospholipid transporter. The requirement of hydrolyzable Mg2+-ATP was demonstrated as being necessary for the rapid transport of aminophospholipids, and the specificity was carefully investigated; however, no proteins were identified initially. In 1989, an ATP-dependent flippase activity in chromaffin granules from bovine adrenal medulla was reported by the Paris laboratory and attributed to the so-called ATPase II [15]. This was the first report of aminophospholipid translocase activity in the inner membranes of the eukaryotic cell. The transport observed was in fact from the lumen to the cytosol of the granules but was classified as a flippase activity. In 1996, P. Williamson and R.A. Schlegel’s groups in the United States showed that this granule flippase was homolog to a yeast ATPase (called Drs2p), and studied a mutant deprived of Drs2 that was unable to flip aminophospholipids [16]. The phospholipid flippase seemed to be discovered.
However, in 1999 and 2003, the groups of T. Graham in the United States [17] and G. van Meer and J. Holthuis in The Netherlands [18] showed that Drs2p is in fact localized in the yeast trans-Golgi and not in the plasma membrane, and that five homologs of this protein exist: two in the plasma membrane (Dnf1p and Dnf2p), two in the trans-Golgi (Dnf3p and Drs2p), and one (Neo1p) in endosomes or cis-Golgi. Furthermore, these P-type ATPases seem to be associated with other proteins playing the role of chaperones (CDC50p) or are necessary for the proper targeting to their final destination of the newly formed proteins [19]. In 2006, P. Natarajan and T. Graham [20] showed a flippase activity with fluorescent lipids in yeast Golgi membranes, which they could attribute to the Drs2p. Interestingly, the triple knockout of the Drs2p homologs in yeast led to viable cells, but they were deprived of endocytic activity [18]. In conclusion, the various P-type ATPases may have different specificities but may also be partially redundant.
Thus, after about 20 years of research in different laboratories throughout the world, it became obvious that the ubiquitous eukaryotic flippase was in reality a combination of several proteins, including four ATPases called P4-ATPase, actually forming a family of five proteins in yeast. In humans, it was predicted from genomic investigation that 14 P4-ATPases were members of the family and could be involved in lipid transport. The purification of specific P4-ATPases and of Drs2p from chromaffin granules or after expression in various systems (yeast and insect cells) is in progress. However, so far the purification has not been achieved on a large enough scale to allow unambiguous tests of lipid transport in reconstituted lipoproteins.
Other membrane proteins were reported to have an ATP-dependent lipid translocation activity and correspond to the so-called floppases (see Fig. I.1) with an ATP-binding cassette (ABC). Suggested originally by C.F. Higgins and M.M. Gottesman in 1992 [21], the laboratories of G. van Meer and of P. Borst in The Netherlands [22] showed in 1996 that the ABC transporter P-glycoprotein, also called MDR1, which is responsible for multidrug resistance and is a serious obstacle in cancer therapy, was able to transport fluorescent phospholipids from the inner monolayer to the outer monolayer of the plasma membrane of eukaryotic cells. The low specificity of the P-glycoprotein suggested that this protein could be involved in the transport of SM and PC toward the outer monolayer of the plasma membrane, hence play an important role in the transmembrane lipid asymmetry of the eukaryotic plasma membrane. Other members of the ABC protein family seemed to be responsible for the specific outward transport of PC in transfected epithelial cells [22]. An important point is that ABC proteins are also found in prokaryotes and could be implicated in lipid translocation in bacteria [23].
Besides ATP-dependent flippases, which were found essentially in the plasma membrane of eukaryotes, other ATP-independent proteins also called flippases were postulated to be in specific organelle membranes (endoplasmic reticulum) and could explain the rapid flip-flop observed by several groups. Their primary function would be to facilitate the transmembrane diffusion of lipids in the membranes specialized in lipid synthesis. Already in 1985, W.R. Bishop and R.M. Bell [24] suggested the existence of ATP-independent flippase, catalyzing the diffusion of PC in the endoplasmic reticulum. Since then, several researchers have attempted to isolate the protein(s) responsible (A. Menon in the United States [25] and A. Herrmann and collaborators in Germany [26]). Other researchers have attempted to prove that any transmembrane protein should accelerate the flip-flop of lipids, making it unnecessary to search for specific proteins (B. de Kruijff and A. Killian and collaborators in The Netherlands [27, 28]). In practice, the identification of the ATP-independent flippase of low lipid specificity seems even more difficult than it is for the ATP-dependent selective flippase, precisely because the test of ATP requirement cannot be used to discover the latter transporter.
BIOLOGICAL ADVANTAGES OF LIPID ASYMMETRY
The complexity involved in the regulation of lipid topology, requiring ATP hydrolysis, raises the question of the biological function(s) of such an elaborate system. Actually, one might rephrase this question differently: The lipid composition of a biological membrane is always a mixture of many different lipids. The actual justification of this fact is not obvious, since a stable lipid bilayer can be achieved in liposomes with a single phospholipid species. So what is the biological advantage of the synthesis of many different lipids? A reasonable hypothesis would be that lipid asymmetry is used to tag the two sides of a membrane and to optimize their functionality, which is obviously different. Indeed, the cell outer environment differs fundamentally from the cytosol.
One of the first indications of the physiological importance of lipid asymmetry came from the observation by A. Schroit and collaborators (United States) who showed in 1983 and 1985 that the presence of a very small percentage of PS (∼1% of the total lipid composition) in the outer monolayer of red cells was used in vivo as a signal of cell aging and led in the blood circulation to the elimination of aged cells by macrophages [29]. These conclusions, which came originally from experiments associated with the introduction of exogenous PS in the outer monolayer of red cells, were confirmed later by the detection of natural PS with fluorescent Annexin V by J.F. Tait and D. Gibson in 1994 [30]. The exposure of PS in the outer monolayer of platelets is also associated with the formation of clots that stop bleeding (R. Zwaal and collaborators [31]).
Thus, lipid flip-flop concerns directly at least two important physiological problems: (1) blood coagulation, which is triggered in vivo by the exposure of PS, a cofactor required for the conversion of prothrombin into thrombin, and (2) elimination of aged and/or apoptotic cells by macrophages. The lipid randomization, that is, loss of lipid asymmetry that is used in vivo as a signal for cell elimination, can be triggered artificially by penetration of calcium ions in the cytosol of platelets, erythrocytes, or lymphocytes with calcium ionophores, and results in “lipid scrambling,” that is, lipid randomization between the two leaflets. This phenomenon is associated with a so far unknown protein named “scramblase.” (Note: Suzuki et al. [40] identified the protein TMEM16F as an essential component for Ca2+-dependent exposure of PS.) A rare but severe disease, called “Scott syndrome,” is characterized by the absence of PS redistribution upon calcium entry and has been investigated by R. Zwaal’s group in The Netherlands [32] and by J.-M. Freyssinet and collaborators [33] in France.
Various severe diseases such as cancer and Alzheimer’s disease were also reported to be accompanied by defects in lipid asymmetry [34]. ABCA1, another lipid transporter of the family of ABC-ATPases, was considered to be responsible for Tangier disease, characterized by impaired efflux of cholesterol and phospholipids from peripheral cells onto apolipoproteins such as Apo A-1. Cholesterol accumulation in macrophages and apolipoprotein degradation lead to tissue deposition of cholesterol esters and increase the risk of arteriosclerosis in patients. G. Chimini and collaborators in Marseilles studied this particular defect associated with a lipid transporter [35]. In humans, several mutated ABC proteins reputed to be responsible for lipid transport are believed to cause metabolism disorders such as Stargardt syndrome (a genetic disease of vision), progressive intrahepatic cholestasis, pseudoxanthoma elasticum, adrenoleukodystrophy, or sitosterolemia.
In 1999, E. Farge and collaborators, in A. Dautry-Varsat’s laboratory, provided evidence of a biological role played by a lipid transporter during the first step of endocytosis [36]. It was shown that the transport of PS and PE from the outer to the inner monolayer by the ATP-dependent flippase is a stimulation of endocytosis and could be the molecular motor of membrane bending involved in the first step of endocytosis. The explanation proposed was that the excess of lipids in one monolayer triggers membrane invagination, as shown in model systems [37]. The yeast knockout experiments mentioned above [18] confirmed that in the absence of flippase proteins, endocytosis was blocked.
There are also reports suggesting that PS is important for fusion; hence, it could be useful in the inner monolayer for exocytosis and not only for the regulation of inner leaflet proteins.
There are certainly many other enzymes that require specific lipids at specific positions in a cell. Actually, the difference in head group of the lipids from the two sides of a membrane is not the only difference between inner and outer leaflets lipids. Indeed, there is evidence of difference in unsaturation, which is associated with differences in membrane viscosity, as observed in erythrocytes with spin-labeled lipids [38] and with fluorescent lipids [39]: The inner monolayer is more fluid; the outer is more rigid, hence more resistant. It is very likely that this feature is associated with the activity of proteins.
When transmembrane lipid asymmetry was demonstrated in red cells and soon after in the plasma membranes of all animals, it was assumed that this feature was a general property of living organisms. This may be true in animal and in plant cells, which are both eukaryotes. But the evidence regarding prokaryotes is limited and often concerns rare lipids.
PROSPECTS
What kind of progress can be expected in a reasonable time? Clearly, the bottleneck for progress in understanding the mechanism of lipid translocation by membrane proteins in eukaryotes has been the difficulty in assigning, isolating, and overexpressing the protein(s) responsible for this process; studying the properties of proteoliposomes; and crystallizing a flippase. Crystallization will be a necessary step for ultimately understanding the mechanism that allows a hydrophobic transmembrane protein to accumulate against a gradient amphiphilic molecule. There are some reports, at low resolution, on the structure of ABC proteins possibly involved in lipid transport. With P4-type ATPases, the data obtained with Ca2+-ATPase can be used as first-order approximation to stimulate the speculations of researchers, but the difference between a lipid and a calcium ion is so large that the detailed analysis of the mechanism is presumptuous. In any case, the determination of the structure and molecular mechanism of a flippase is a challenge for the coming years. It is therefore an objective that cannot be forsaken. Progress in the molecular biology and purification of the P4-type ATPases will lead to this achievement.
Other objectives are as follows:
1. isolation of protein(s) responsible for ATP-independent rapid lipid flip-flop in the endoplasmic reticulum;
2. isolation of protein(s) responsible for calcium-induced lipid scrambling (scramblase);
3. deeper understanding of all the consequences of lipid asymmetry, including recognition of the diseases caused specifically by a defect (impairment) in flippase activity.
ORGANIZATION OF THIS BOOK
As shown in the Table of Contents of this book, each chapter concentrates on one particular aspect of lipid asymmetry in biomembranes. However, we are not yet in a situation to give a complete and rational picture. As a consequence, one of the main difficulties in assembling this book was to choose a rational order for the chapters. Although the various chapters are closely linked to each other, there were no compelling reasons to decide which subjects deserved to be first or second. Hence, some repetition is unavoidable and the order of the chapters is rather arbitrary. Nevertheless, we must apologize for this weakness. On the other hand, each chapter can stand alone and does not necessarily require the reading of other chapters.
ACKNOWLEDGMENTS
We would like to express our gratitude to the contributing authors, in particular for being so flexible and sympathetic. Many of the contributing authors belonged to an international research and training network, “Flippases,” which was funded by the European Union from 2005 to 2008. This network provided an important trigger for assembling the book. We thank Professor Sophie Cribier (Paris) and Professor Daniel Picot (Paris) for their help in speeding up the final steps for assembling the book. Finally, we are very much indebted to Anita Lekhwani, Senior Acquisitions Editor at John Wiley and Sons, and to Catherine Odal, Assistant to the Senior Acquisitions Editor, for their efficient collaboration and support as well as for being flexible and handling unforeseen problems pleasantly.
PHILIPPE F. DEVAUX
ANDREAS HERRMANN
November 2010
REFERENCES
1 O. Mouritsen, Lipid—As a Matter of Fat. The Merging Science of Lipidomics, Springer, Berlin, 2005.
2 C. Nakada, K. Ritchie, Y. Oba, M. Nakamura, Y. Hotta, R. Iino, R. S. Kasai, K. Yamaguchi, T. Fujiwara, A. Kusumi, Nat. Cell Biol. 2003, 5, 626–632.
3 R. P. Rand, V. Luzzati, Biophys. J. 1968, 8, 125–137.
4 V. Luzzati, F. Reiss-Husson, E. Rivas, T. Gulik-Krzywicki, Ann. N.Y. Acad. Sci. 1966, 137, 409–413.
5 S. J. Singer, G. L. Nicolson, Science 1972, 175, 720–731.
6 K. Simons, E. Ikonen, Nature 1997, 387, 569–572.
7 M. S. Bretscher, Science 1973, 181, 622–629.
8 A. J. Verkleij, R. F. A. Zwaal, B. Roelofsen, P. Comfurius, D. Kastelijn, L. L. M. van Deenen, Biochim. Biophys. Acta 1973, 323, 178–193.
9 R. F. A. Zwaal, B. Roelofsen, P. Comfurius, L. L. M. van Deenen, Biochim. Biophys. Acta 1975, 406, 83–96.
10 J. A. F. Op den Kamp, Biochemistry 1979, 48, 47–71.
11 R. D. Kornberg, H. M. McConnell, Biochemistry 1971, 10, 1111–1120.
12 M. Seigneuret, P. F. Devaux, Proc. Natl. Acad. Sci. U.S.A. 1984, 81, 3751–3755.
13 D. L. Daleke, W. H. Huestis, Biochemistry 1985, 24, 5406–5416.
14 J. Connor, A. J. Schroit, Biochemistry 1987, 26, 5099–5105.
15 A. Zachowski, J. P. Henry, P. F. Devaux, Nature 1989, 340, 75–76.
16 X. J. Tang, M. S. Halleck, R. A. Schlegel, P. Williamson, Science 1996, 272, 1495–1497.
17 C.-Y. Chen, M. F. Ingram, P. H. Rosal, T. R. Graham, J. Cell Biol. 1999, 147, 1223–1236.
18 T. Pomorski, R. Lombardi, H. Riezman, P. F. Devaux, G. van Meer, J. C. Holthuis, Mol. Biol. Cell 2003, 14, 1240–1254.
19 K. Saito, K. Fujimura-Kamada, N. Furuta, U. Kato, M. Umeda, K. Tanaka, Mol. Biol. Cell 2004, 15, 3418–3432.
20 P. Natarajan, T. R. Graham, Methods 2006, 39, 163–168.
21 C. F. Higgins, M. M. Gottesman, Trends Biochem. Sci. 1992, 17, 18–21.
22 A. van Helvoort, A. J. Smith, H. Sprong, I. Fritzsche, A. H. Schinkel, P. Borst, G. van Meer, Cell 1996, 87, 507–517.
23 A. Pohl, P. F. Devaux, A. Herrmann, Biochim. Biophys. Acta 2005, 1733, 29–52.
24 W. R. Bishop, R. M. Bell, Cell 1985, 42, 51–60.
25 A. Menon, W. E. Watkins, III, S. Hrafnsdóttir, Curr. Biol. 2000, 10, 241–252.
26 S. Vehring, L. Pakkiri, A. Schroer, N. Alder-Baerens, A. Herrmann, A. K. Menon, T. Pomorski, Eukaryot. Cell 2007, 6, 1625–1634.
27 M. A. Kol, A. I. P. M. de Kroon, J. A. Killian, B. de Kruijff, Biochemistry 2004, 43, 2673–2681.
28 M. A. Kol, A. I. P. M. de Kroon, D. T. S. Rijkers, J. A. Killian, B. de Kruijff, Biochemistry 2001, 40, 10500–10506.
29 A. J. Schroit, J. W. Madsen, Y. Tanaka, J. Biol. Chem. 1985, 260, 5131–5138.
30 J. F. Tait, D. Gibson, J. Lab. Clin. Med. 1994, 123, 741–748.
31 E. M. Bevers, P. Comfurius, R. F. A. Zwaal, Biochim. Biophys. Acta 1983, 736, 57–66.
32 E. M. Bevers, T. Wiedmer, P. Comfurius, S. J. Shattil, H. J. Weiss, R. F. A. Zwaal, P. J. Sims, Blood 1992, 79, 380–388.
33 N. Bettache, P. Gaffet, N. Allegre, L. Maurin, F. Toti, J.-M. Freyssinet, A. Bienvenue, Br. J. Haematol. 1998, 101, 50–58.
34 A. Castegna, C. M. Lauderback, H. Mohmmad-Abdul, D. A. Butterfield, Brain Res. 2004, 1004, 193–197.
35 Y. Hamon, C. Broccardo, O. Chambenoit, M.-F. Luciani, F. Toti, S. Chaslin, J.-M. Freyssinet, P. F. Devaux, J. Neish, D. Marguet, G. Chimini, Nat. Cell Biol. 2000, 2, 399–406.
36 E. Farge, D. M. Ojcius, A. Subtil, A. DautryVarsat, Am. J. Physiol. Cell Physiol. 1999, 45, C725–C733.
37 E. Farge, P. Devaux, Biophys. J. 1992, 61, 347–357.
38 M. Seigneuret, A. Zachowski, A. Herrmann, P. F. Devaux, Biochemistry 1984, 23, 4271–4275.
39 G. Morrot, S. Cribier, P. F. Devaux, D. Geldwerth, J. Davoust, J. F. Bureau, P. Fellmann, P. Herve, B. Frilley, Proc. Natl. Acad. Sci. U.S.A. 1986, 83, 6863–6867.
40 Suzuki et al., Nature 2010, 468, 834–838.
LIST OF CONTRIBUTORS
Krishnakumar Balasubramanian, University of Pittsburgh, Pittsburgh, PA 15219; Email: [email protected]
Edouard M. Bevers, Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, The Netherlands; Email: [email protected]
Adam Blanchard, School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington Campus, College Road, Sutton Bonington, Leicestershire LE12 5RD, UK
Richard Callaghan, Nuffield Department of Clinical Laboratory Sciences, John Radcliffe Hospital, University of Oxford, Oxford, UK; Email: [email protected]
Giovanna Chimini, Centre d’Immunologie de Marseille-Luminy, INSERM-CNRS-Université de La Méditerranée, Parc Scientifique de Luminy, 13288, Marseille, France; Email: [email protected]
Shelley M. Cook, Department of Biochemistry and Molecular Biology, Medical Sciences, Bloomington, Indiana University School of Medicine, Bloomington, IN 47405
David L. Daleke, Department of Biochemistry and Molecular Biology, Medical Sciences, Bloomington, Indiana University School of Medicine, Bloomington, IN 47405; Email: [email protected]
Philippe F. Devaux, Institut de Biologie Physico-Chimique, 13 rue Pierre et Marie Curie 75005 Paris , France; Email: [email protected]
Jean-Marie Freyssinet, U. 770 INSERM, Hôpital de Bicêtre, Le Kremlin-Bicêtre, France; Faculté de Médecine, Université Paris-Sud, Le Kremlin-Bicêtre, France; and Faculté de Médecine, Institut d’Hématologie & Immunologie, Université de Strasbourg, Strasbourg, France; Email: [email protected]
Todd R. Graham, Department of Biological Sciences, Vanderbilt University, Nashville, TN 37235; Email: [email protected]
Per Haberkant, EMBL, Heidelberg, Germany
Andreas Herrmann, Department of Biology, Humboldt-University Berlin, Invalidenstr. 42, D-10115 Berlin, Germany; Email: [email protected]
Joost C.M. Holthuis, Department of Membrane Enzymology, Bijvoet Center and Institute of Biomembranes, Utrecht University, 3584 CH Utrecht, The Netherlands; Email: [email protected]
Frans A. Kuypers, Children’s Hospital Oakland Research Institute, 5700 Martin Luther King Way, Oakland, CA 94609; Email: [email protected]
Ke Liu, NIH Chemical Genomics Center, Bethesda, MD 20892
Iván López-Montero, Departamento de Química Física I, Universidad Complutense de Madrid, 28040 Madrid, Spain; Email: [email protected]
Anant K. Menon, Department of Biochemistry, Weill Cornell Medical College, New York, NY 10065
Peter Müller, Department of Biology, Humboldt-University Berlin, Invalidenstr. 42, D-10115 Berlin, Germany; Email: [email protected]
Baby-Periyanayaki Muthusamy, Department of Biological Sciences, Vanderbilt University, Nashville, TN 37235
Paramasivam Natarajan, Department of Biological Sciences, Vanderbilt University, Nashville, TN 37235
Sylvia Neumann, Department of Cell Biology, The Scripps Research Institute, La Jolla, CA
Petra H.M. Niesten, Nuffield Department of Clinical Laboratory Sciences, John Radcliffe Hospital, University of Oxford, Oxford, UK
Nina Ohlwein, Department of Biology, Humboldt University Berlin, Invalidenstr. 42, D-10115 Berlin, Germany; Email: [email protected]
Anna Pia Plazzo, Department of Biology, Humboldt-University Berlin, Invalidenstr. 42, D-10115 Berlin, Germany
Naomi L. Pollock, Nuffield Department of Clinical Laboratory Sciences, John Radcliffe Hospital, University of Oxford, Oxford, UK
Thomas G. Pomorski, Department of Plant Biology and Biotechnology, Faculty of Life Sciences, University of Copenhagen, DK-1871 Frederiksberg C, Denmark
Cyril Rauch, School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington Campus, College Road, Sutton Bonington, Leicestershire LE12 5RD, UK; Email: [email protected]
Alan J. Schroit, Department of Pharmacology, The University of Texas Southwestern Medical Center, Dallas, TX 75390; Email: [email protected]
Eric Soupene, Children’s Hospital Oakland Research Institute, 5700 Martin Luther King Way, Oakland, CA 94609
Florence Toti, U. 770 INSERM, Hôpital de Bicêtre, Le Kremlin-Bicêtre, France; Faculté de Médecine, Université Paris-Sud, Le Kremlin-Bicêtre, France; and Faculté de Médecine, Institut d’Hématologie & Immunologie, Université de Strasbourg, Strasbourg, France
Gerrit van Meer, Faculty of Science, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands; Email: [email protected]
Marisela Vélez, Instituto de Catálisis y Petroleoquímica, Consejo Superior de Investigaciones Científicas, 28049 Madrid, Spain; and IMDEA Nanociencia, Facultad de Ciencias, Universidad Autonóma de Madrid, 28049 Madrid, Spain;
Patricia M. Verhulst, Department of Membrane Enzymology, Bijvoet Center and Institute of Biomembranes, Utrecht University, 3584 CH Utrecht, The Netherlands
Patrick L. Williamson, Department of Biology, Amherst College, Amherst, MA; Email: [email protected]
Alain Zachowski, Laboratory of “Physiologie Cellulaire et Moléculaire des Plantes,” Université Pierre et Marie Curie—Paris 6 (UR 5) and Centre National de la Recherche Scientifique (EAC 7180); Email: [email protected]
Ana Zarubica, Centre d’Immunologie de Marseille-Luminy, INSERM-CNRS-Université de La Méditerranée, Parc Scientifique de Luminy, 13288, Marseille, France
Xiaoming Zhou, Department of Biological Sciences, Vanderbilt University, Nashville, TN 37235





























