Wound Healing E-Book
158,99 €
Mehr erfahren.
- Herausgeber: John Wiley & Sons
- Kategorie: Wissenschaft und neue Technologien
- Sprache: Englisch
A comprehensive resource on the recent developments of stem cell use in wound healing With contributions from experts in the field, Wound Healing offers a thorough review of the most recent findings on the use of stem cells to heal wounds. This important resource covers both the basic and translational aspects of the field. The contributors reveal the great progress that has been made in recent years and explore a wide range of topics from an overview of the stem cell process in wound repair to inflammation and cancer. They offer a better understanding of the identities of skin stem cells as well as the signals that govern their behavior that contributes to the development of improved therapies for scarring and poorly healing wounds. Comprehensive in scope, this authoritative resource covers a wealth of topics such as: an overview of stem cell regeneration and repair, wound healing and cutaneous wound healing, the role of bone marrow derived stems cells, inflammation in wound repair, role and function of inflammation in wound repair, and much more. This vital resource: * Provides a comprehensive overview of stem cell use in wound healing, including both the basic and translational aspects of the field * Covers recent developments and emerging subtopics within the field * Offers an invaluable resource to clinical and basic researchers who are interested in wound healing, stem cells, and regenerative medicine * Contains contributions from leading experts in the field of wound healing and care Wound Healing offers clinical researchers and academics a much-needed resource written by noted experts in the field that explores the role of stem cells in the repair and restoration of healing wounds.
Sie lesen das E-Book in den Legimi-Apps auf:
Seitenzahl: 681
Veröffentlichungsjahr: 2017
Ähnliche
Wound Healing
Stem Cells Repair and Restorations, Basic and Clinical Aspects
Edited by
Kursad Turksen
This edition first published 2018 © 2018 John Wiley & Sons, Inc.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at http://www.wiley.com/go/permissions.
The right of Kursad Turksen to be identified as the editor of this work has been asserted in accordance with law.
Registered OfficeJohn Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA
Editorial Office111 River Street, Hoboken, NJ 07030, USA
For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com.
Wiley also publishes its books in a variety of electronic formats and by print-on-demand. Some content that appears in standard print versions of this book may not be available in other formats.
Limit of Liability/Disclaimer of WarrantyThe publisher and the author make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties; including without limitation any implied warranties of fitness for a particular purpose. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for every situation. In view of on-going research, equipment modifications, changes in governmental regulations, and the constant flow of information relating to the use of experimental reagents, equipment, and devices, the reader is urged to review and evaluate the information provided in the package insert or instructions for each chemical, piece of equipment, reagent, or device for, among other things, any changes in the instructions or indication of usage and for added warnings and precautions. The fact that an organization or website is referred to in this work as a citation and/or potential source of further information does not mean that the author or the publisher endorses the information the organization or website may provide or recommendations it may make. Further, readers should be aware that websites listed in this work may have changed or disappeared between when this works was written and when it is read. No warranty may be created or extended by any promotional statements for this work. Neither the publisher nor the author shall be liable for any damages arising here from.
Library of Congress Cataloguing-in-Publication Data applied for.
Hardback: 9781119282488
Cover Design: Wiley Cover Image: © Designua/Shutterstock
CONTENTS
List of Contributors
Chapter 1 Stem Cell Regeneration and Repair – Overview
Introduction
Overview of Skin Wound Healing
Stem Cell Definition: History
Stem Cells in Skin Homeostasis and Repair
Therapies for Wound Healing and Scarring that Target or Utilize Stem Cells
Conclusion
References
Chapter 2 Cadherins as Central Modulators of Wound Repair
Introduction
Epithelial Cadherins and Adherens Junctions: Structure and Functions
Regulation of Adherens Junctions by Rho GTPases
Crosstalk Between Adherens Junctions and Other Signaling Pathways
Desmosomal Cadherins and Desmosomes: Structure and Functions
Biomechanical Sensing Properties of Cadherin-Based Cell–Cell Adhesions and Mechanotransduction
Biochemical Signaling and Actin Cytoskeleton Dynamics vis-à-vis Cadherin-Based Cell–Cell Adhesions and Wound Healing
Crosstalk of Cadherins and Cell–Extracellular Matrix Adhesions
Additional Roles and Regulatory Mechanisms of Adherens Junctions During Wound Healing
Desmosome Regulation and Roles in Wound Healing
Conclusions and Perspectives
Acknowledgments
References
Chapter 3 Tight Junctions and Cutaneous Wound Healing
Introduction
Tight Junctions in Epidermal Wound Healing
TJs and TJ Proteins in Wound Healing of Other Epithelia
Conclusion
References
Chapter 4 The Role of Microvesicles in Cutaneous Wound Healing
Microvesicles
Involvement of Microvesicles in Cutaneous Wound Healing
Potential Implications of Microvesicles in Pathological Wound Healing
Conclusion
Acknowledgments
References
Chapter 5 Wound Healing and Microenvironment
Introduction
The Mechanism of Wound Healing
The Role of the Microenvironment During Wound Healing
Conclusion
References
Chapter 6 Wound Healing and the Non-cellular Microenvironment
Introduction
The Normal Tissue Microenvironment
Microenvironment Changes During Wound Healing
Therapeutic Approaches Targeting the Microenvironment
Outlook
References
Chapter 7 Contribution of Adipose-Derived Cells to Skin Wound Healing
Introduction
Adipose Depots, Their Location and Functionality
Adipose Tissue Composition
Adipose-Derived Cells
Mammalian Skin Histology
The Skin Wound Healing Process
Skin Wound Models
Rodent Outcomes with Adipose-Derived Cells
Large Animal Outcomes with Adipose-Derived Cells
Conclusions and Future Directions
Disclosures
References
Chapter 8 Role of Bone Marrow-Derived Stem Cells in Wound Healing
Introduction
What Are Mesenchymal Stem Cells?
Wound Healing
Chronic Wounds
Conclusion
References
Chapter 9 Role of Vitamin D and Calcium in Epidermal Wound Repair
Introduction
Vitamin D Signaling and the Role of the Vitamin D Receptor (VDR)
Calcium Signaling and Role of the Calcium Sensing Receptor (Casr)
Ctnnb1 (β-Catenin) Signaling
Calcium and Vitamin D Signaling During Wound Repair
Conclusions
References
Chapter 10 Oral Mucosal Healing
Introduction
Environment
Oral Mucosa Architecture
Fibroblasts
Keratinocytes
Inflammation in Oral Mucosal Wounds
Angiogenesis
Scar Formation
Stem Cells
Summary
References
Chapter 11 Role of Adipose-Derived Stem Cells in Wound Healing: An Update from Isolation to Transplantation
Introduction
Adipose Tissue-Derived Mesenchymal Stem Cells
ADSCs in Wound Healing
Strategies to Improve ADSC Potential in Wound Healing
Clinical Studies
Future Directions
Conflict of Interests
References
Chapter 12 The Hair Follicle as a Wound Healing Promoter and Its Application in Clinical Practice
Introduction
Clinical Evidence of the Importance of Hair Follicles in Wound Healing
Basic Research Evidence Highlights a Key Role of HF Epithelium and Mesenchyme in the Initial Wound Healing Response
Conclusions
Acknowledgments
References
Chapter 13 Impaired Wound Healing in Diabetic Ulcers: Accelerated Healing Through Depletion of Ganglioside
Impaired Healing in Diabetic Wounds
The Insulin/IGF1 Signaling Axis in Normal and Diabetic Wounds
GM3 Mediates TNF- and Glucose-Induced Insulin Resistance in Diabetes
GM3 is a Driver of Impaired Wound Healing in Type 2 Diabetic Mice
Confirmation that IGF1R is the Primary Target of GM3 in Cultured KCs
The Role of GM3 in Impairing Wound Healing May Extend Beyond Epidermis
Summary
Acknowledgments
References
Chapter 14 Inflammation in Wound Repair: Role and Function of Inflammation in Wound Repair
Introduction
Role of the Antimicrobial Host Defense System in Wound Repair
Cytokines and Chemokines
Nucleic Acids and Other DAMPS and PAMPS
Commensals and Pathogens in Wound Inflammation
Dysregulation of Inflammation Results in Non-Healing Wounds
Concluding Remarks
References
Chapter 15 Inflammation, Wound Healing, and Fibrosis
Introduction
Tissue Injury, Wound Healing, and Innate Immune Defense
Myofibroblasts, Normal Wound Healing, and Fibrosis
Primary Cell Type Necessary for Fibrosis
Development of Apoptosis-Resistant Mesenchymal Phenotypes in Pulmonary Fibrosis
Conclusion
Acknowledgments
References
Chapter 16 The Potential Role of Photobiomodulation and Polysaccharide-Based Biomaterials in Wound Healing Applications
Introduction
PBM Mediated Wound Healing
Polysaccharides Mediated Wound Healing
Combination of PBM and Polysaccharides in Wound Healing
Future Perspectives and Conclusion
Conflict of Interest
Acknowledgments
References
Chapter 17 Is Understanding Fetal Wound Repair the Holy Grail to Preventing Scarring?
Introduction
Inflammation
Growth Factors
Extra Cellular Matrix (ECM)
Wound Closure
Conclusion
References
Chapter 18 Inflammation and Cancer
Introduction
Tissue Injury, Fibrosis, and Tumor Microenvironment
Inflammation and Tumors
Concluding Remarks and Therapeutic Strategies
Acknowledgments
References
Index
EULA
List of Tables
Chapter 3
Table 3.1
Chapter 4
Table 4.1
Table 4.2
Table 4.3
Table 4.4
Chapter 10
Table 10.1
Chapter 11
Table 11.1
Chapter 16
Table 16.1
Table 16.2
List of Illustrations
Chapter 1
Figure 1.1
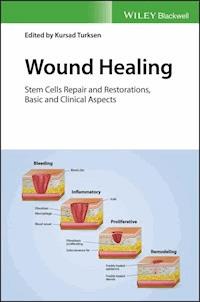
There are three classic stages of wound repair: (a) inflammation, (b) new tissue formation, and (c) remodeling.
(a) Inflammation. This stage lasts until about 48 h after injury. Depicted is a skin wound at about 24–48 h after injury. The wound is characterized by a hypoxic (ischaemic) environment in which a fibrin clot has formed. Bacteria, neutrophils, and platelets are abundant in the wound. Normal skin appendages (such as hair follicles and sweat duct glands) are still present in the skin outside the wound. (b) New tissue formation. This stage occurs about 2–10 days after injury. Depicted is a skin wound at about 5–10 days after injury. An eschar (scab) has formed on the surface of the wound. Most cells from the previous stage of repair have migrated from the wound, and new blood vessels now populate the area. The migration of epithelial cells can be observed under the eschar. (c) Remodeling. This stage lasts for a year or longer. Depicted is a skin wound about 1–12 months after repair. Disorganized collagen has been laid down by fibroblasts that have migrated into the wound. The wound has contracted near its surface and the widest portion is now the deepest. The re-epithelialized wound is slightly higher than the surrounding surface and the healed region does not contain normal skin appendages. (Reproduced from Gurtner et al. [1], with permission from Nature Publishing Group.)
Figure 1.2
Masson's trichrome staining of the interface between normal (left) and scarred (right) dorsal skin in the adult mouse.
Normal skin contains hair follicles and other dermal appendages. Scarred skin does not contain these appendages and the epidermis is flattened. Note that that scarred dermis is thicker than the normal dermis. Scale bar, 500 μm.
Figure 1.3
Different approaches to lineage tracing.
(a) Direct observation, as pioneered by Whitman and colleagues (exemplified by a plate from Conklin, 1905) [14]. (b) Schematic showing agar chips with vital dyes applied on to the surface of an early stage amphibian embryo (top). These dyes label regions within later stage embryos (bottom) (based on Vogt, 1929; adapted from Gilbert, 2000 [15, 16]). (c) Use of soluble carbocyanine dyes to fate map chick neural crest. (Reproduced from Serbedzija et al., 1989, with permission from The Company of Biologists Ltd [17].) (d) Whole-mount of mouse epidermis showing DNA label-retaining stem cells in the hair follicle bulge. (Reproduced from Braun et al., 2003 [18].) Red: keratin 14; green: BrdU. Scale bar: 100 μm. (e)
LacZ
retroviral vector introduced into rat retinal cells (upper panel) and subsequently tracked in the reconstituted retina. (Reproduced from Price et al., 1987, with permission from C. Cepko [19].) (f) Schematic showing Spemann and Mangold's organizer experiment, which was performed by grafting tissues between amphibian embryos (adapted from Grove, 2008 [20]). (g and h) Adult mouse chimeras from GFP-positive and -negative mice. (g) Whole-mount of lung (reproduced from Giangreco et al., 2009 [21]). (h) Histology of skin tumor (reproduced from Arwert et al., 2010 [22]). The GFP-positive region in (h) is brown. (Reproduced with permission from Kretzschmar and Watt [13].)
Figure 1.4
Heterogeneity of epidermal and hair follicle stem cells.
Skin epithelia feature distinct stem cell populations both in epidermis and, most prominently, in hair follicles. Epithelial stem cells in different microanatomical locations have different lineage potentials. Stem cells in the follicular infundibulum and interfollicular epidermis physiologically are restricted to epidermal fate. Interfollicular epidermal stem cells can be identified as slow-cycling in label retention studies, but distinct markers remain elusive. The isthmus and junctional zone of hair follicles harbor several distinct epithelial cell populations. Most prominent among them are Lrig1+ (yellow), Gli1+, and Lgr6+ stem cells (green), all of which physiologically maintain the isthmus and contribute to sebaceous gland, infundibulum and in some instances to interfollicular epidermis. Blimp1 identifies unipotent sebaceous gland progenitors (orange). The bulge stem cells (blue) normally contribute to all hair follicle lineages and can be identified based on the expression of Krt15, CD200, Lgr5, CD34, Sox9, Lhx2, Tcf3 and Nfatc1. The secondary germ of telogen hair follicles (purple) contains committed hair follicle-fated progenitors that express CD200, Gli1 and Lgr5. (Reproduced from Plikus et al. [9], with permission from Elsevier.)
Chapter 5
Figure 5.1
Wound healing stages.
Figure 5.2
Growth factors involved in wound healing.
Chapter 8
Figure 8.1
BMDSCs can be isolated from bone marrow and separated from hematopoietic stem cells by the effect of adhesion. These cells can be expanded in culture and characterized by STRO-1 positivity and CD45 negativity. They can differentiate all cells in culture such as osteoblast, which are identified by alkaline phosphate/von kossa (ALP/VK) and osteonectin (ON). Magnification × 400.
Figure 8.2
BMDSCs can be differentiated to osteoblast in culture and therefore can be functional for bone healing by (a) macroscopic and (b) microscopic presentations. Magnification × 400.
Figure 8.3
BMDSCs can be used for skin wound healing, which starts with hypoxia and alteration of the metabolism. For the first three days, organization of inflammation by the cells and factors occur. In the next two weeks proliferation starts. After two weeks matrix and adhesion production produce self-renewal and re-epithelialization for months to accelerate wound healing. Magnification × 400.
Chapter 9
Figure 9.1
A cartoon of the interfollicular epidermis and hair follicle
showing the different stem cell niches and the markers generally used to identify the different stem cell populations.
Figure 9.2
A model of the E-cadherin/catenin complex and its role in differentiation.
Both 1,25(OH)
2
D/VDR and calcium/Casr are required for the formation of the E-cadherin/catenin complex in the plasma membrane. This complex is comprised of E-cadherin, 3 catenins (Ctnnb1 or β-catenin, Ctnna1 or α-catenin (not shown), and Ctnnd1 or p120), and two enzymes, phosphatidyl inositol 3 kinase (PI3K) and phosphatidyl inositol phosphate 5 kinase (PIP5K1α). Ctnnd1 maintains the integrity of the complex. Ctnna1 links the complex to the underlying cytoskeleton, Ctnnb1 anchors the enzymes and when released from the complex enters the nucleus to stimulate proliferation and activation of the stem cells. PIP5K1α and PI3K sequentially phosphorylate PIP to PIP3. PIP3 activates phospholipase C-γ1 (Plcg1), which in turn hydrolyzes PIP2 to form inositol tris phosphate (IP3) and diacylglycerol (DAG). IP3 stimulates the release of calcium from internal stores; DAG along with calcium activates protein kinase Cα. PIP3 also serves as a means of attracting Akt to the membrane, where it is activated by phosphatidyl inositol dependent kinase 1 (PDK1).
Figure 9.3
A model of vitamin D and calcium signaling in keratinocytes.
1,25(OH)
2
D either from the blood or within the keratinocyte itself binds to the VDR and translocates to the nucleus. For some genes VDR interacts with Ctnnb1 to promote the transcription of genes involved with calcium and Ctnnb1 signaling, leading to stem cell activation, cell migration enabling re-epithelialization, and differentiation of the epidermis covering the wound. Among the genes induced by 1,25(OH)
2
D/VDR are the phospholipase Cs (PLC) and Casr. Casr translocates to the plasma membrane when the ambient calcium concentration is sufficient. The Casr then activates PLC-β and src family kinases (src/fyn). These kinases, by phosphorylating the catenins, enables them to bind to E-cadherin to form the E-cadherin/catenin complex described in the legend to Figure 2, leading to the sequential phosphorylation of PIP to PIP3. PIP3 activates PLC-γ1, which activates calcium channels in the membrane and hydrolyzes PIP3 to IP3 and diacylglycerol (DG). The release of calcium from internal stores by IP3 along with DG activates protein kinase Cα (PKCα), which, by phosphorylation of desmosomes, converts them to a less adhesive form enabling cell migration.
Chapter 10
Figure 10.1
(a) Histology of wound healing in the oral mucosa and skin. Stained wound samples show that at 24 hours postwounding, the oral mucosal wound is almost completely re-epithelialized, while the equivalently sized skin wound is less than 25% closed. By 60 hours postwounding the oral mucosal wound is 100% closed while the skin wound is less than 50% closed. This difference in re-epithelialization rate is quantified in (b). (Reproduced from Schrementi et al. 2008 [8], with permission from John Wiley & Sons.)
Chapter 12
Figure 12.1
Photograph published in Bishop's seminal paper.
(a) The healing response of wounds made at different depths in his forearm. (b) The discrete mounds of granulation tissue so spaced as to suggest that they correspond to the loci of partially destroyed hair follicles. (c) A burn in which all hair follicles survived; some of these have caps of epithelium over them, which shows as white spots. (d) Skin removed with knife almost to subcutaneous tissue. Dark areas are granulations not yet covered with epithelium.
Figure 12.2
Showing how quickly wounds heal on the scalp.
(a) Hair transplant patient with 700 follicular unit extractions that left circular residual wounds of 1 mm in diameter and 3–4 mm in depth. (b) Three days after surgery the wounds are almost completely healed.
Figure 12.3
A number of mechanisms that may underlie the observed effects of the hair follicle as a wound healing promoter.
(1) Hair follicle-derived epidermal stem/precursor cells are recruited immediately after wounding for transient closure, newly formed epidermis being later replaced by new layers generated from interfollicular epidermal precursors. (2) The perifollicular area is a reservoir for mast cells and macrophages and these cells might have a capital role in recruiting immune cells and possibly mesenchymal stromal cells through immunomodulatory cytokine secretion. (3) Hair follicles, as other epidermal annexes, are supported by a neurovascular unit. Paracrine signals and cell precursors derived from the hair follicle may promote angiogenesis and reinnervation of the wounded area. (4) Mesenchymal precursors resident at the dermal papilla or dermal sheath might also be recruited at later stages of wound healing for dermal and extracellular matrix (ECM) remodeling.
Figure 12.4
Contribution of HF-derived cell progeny to the newly generated wound epidermis.
Different populations of HF-derived cells have been genetically traced to determine commitment of their progeny to wound closure. (a) Tracing of
Krt1-15-CrePR*
mice (which labeled approximately 70% of bulge and 50% of non-bulge epidermal cells before wounding) 15 days postinjury showed radial streams of HF-derived cell progeny directed to the wound center [50]. (b) A similar picture was observed two weeks after wounding of induced
Gli1-GIFM
mice in adult telogen (transgenic mice allowing genetic inducible fate mapping to mark and follow Gli1+ cells [26]). (c) Schematic drawing showing how HF-derived cells proliferate inwards in radial streams. (d) Complete replacement of unlabeled cells in the wound (dotted line) by those expressing dnMAML (green), 17 days after injury in K14;dnMAML mice [29]. (Reproduced with permission from Taylor & Francis.)
Figure 12.5
Venous ulcer of a patient from the pilot clinical trial
. The lower quadrant corresponded to the experimental area that received the scalp punch grafts (experimental area) and the top was the control area without grafts.
Figure 12.6
A chronic ulcer transplanted with scalp (hairy) and abdominal (non-hairy) punch grafts.
Numbers on the panels indicate weeks 0, 4, 8, 12, 15, and 18 postintervention. The line divides the ulcer in the experimental and control area. Scalp punch grafts transplanted into the anterior half of the ulcer induced better healing than abdominal punch grafts transplanted into the posterior half.
Figure 12.7
(a) Donor harvesting of scalp punch grafts using a 2 mm punch. (b) Skin grafts are easily released from the subcutaneous tissue and extracted with fine tip forceps. (c) Residual scalp wounds immediately after graft extraction. (d) Graft implantation into the ulcer bed.
Figure 12.8
Once harvested from the donor area, the punch grafts are placed in physiologic saline until their insertion into the wound bed.
Figure 12.9
The insertion of the follicular unit grafts is made with the “stick and place” method: a tiny slit is made with a 19 gauge needle and the graft is inserted with fine-tip forceps. This is a placement method normally used by hair transplant surgeons.
Figure 12.10
A close-up photo of a chronic ulcer with hair punch grafts inserted in the upper portion of the wound bed.
Chapter 13
Figure 13.1
Comparing diabetic wound healing to normal wound healing.
(a) Diabetic keratinocytes at the ulcer edge show increased expression of GM3 and impaired responses to growth factor stimulation, including of the insulin/IGF-1 signaling axis, with delayed wound closure. (b) Overexpression of GM3 inhibits the insulin/IGF-1 signaling axis in diabetic keratinocytes, which slows KC migration and re-epithelialization of scratch wounds
in vitro
.
Chapter 15
Figure 15.1
Schematic presentation of changes in cellular events and matrix events during the course of wound healing and fibrosis. (Adapted from Misra et al.,
IJCB
, 2015.)
Many of the biological processes mediated by hyaluronan (HA) are crucial for wound healing and fibrosis. After injury, wound healing follows a tightly regulated sequence of events. These phases are inflammation, granulation tissue formation, proliferation, re-epithelization, and remodeling. In the early phases, high molecular weight HA is degraded by reactive oxygen species from activated granulocytes and by hyaluronidases secreted from platelets. Then monocytes secrete inflammatory mediators, which attract additional inflammatory cells. Keratinocytes become activated to migrate, proliferate, and to synthesize HA. As a result the LMW degradation products are active inducers of angiogenesis and inflammation. At later stages the interim matrix becomes supplemented with newly synthesized HMW HA, which contributes to tissue remodeling. During repetitive injury, the repairing processes are hindered and the keratinocytes, the endothelial cells, and the smooth muscle cells of the blood vessel interacting with neutrophils and macrophages orchestrate increased cytokine-mediated signaling that augments HA-CD44 signaling, excess collagen production, and fibrosis.
Figure 15.2
Chronic inflammation and persistent infectious stimuli initiate and sustain the fibrotic process.
Persistent extrinsic infections via bacteria, viruses, fungi, and multicellular parasites induce tissue injury. Host responses mediated by the innate immune system facilitate pathogen-associated molecular patterns recognized by the pattern recognition receptors. The combined inflammatory and immune systems promote efforts of various immune and non-immune cells including T cells, B cells, activated macrophages (M1 macrophages), dendritic cells, neutrophils, epithelial cells, and fibroblasts that contribute to the chronic inflammatory response. In the physiological wound healing, Th1 cytokine contributes to an effective immune response against most pathogens and clears the affected tissue debris and dead cells, and then normal healing proceeds and inflammations resolve. In normal wound healing, under mechanical stress, fibroblasts will differentiate into proto-myofibroblasts, which form cytoplasmic actin-containing stress fibers that terminate in fibronexus adhesion complexes. Proto-myofibroblasts also express and organize cellular fibronectin, including the ED-A splice variant, at the cell surface and can generate contractile forces. However, when pathogens are not eliminated, various survival strategies lead to the persistence of pathogens or the persistence of their byproducts in the host. In the presence of mechanical stress, TGFβ1 and TGFβ1-mediated ED-A-induced fibronectin production promotes the modulation of proto-myofibroblasts into differentiated myofibroblasts that are characterized by the
de novo
expression of α-smooth muscle actin that contributes to stress fibers and large fibronexus adhesion complexes. This ineffective response to heal the wound is often twisted toward the Th2 cytokine pattern. The resulting fibrotic response might be a consequence of the myofibroblast in turn facilitating the persistence of pathogens and their byproducts, thereby allowing this vicious cycle to continue.
Figure 15.3
Origin of myofibroblasts.
Differentiated myofibroblasts are the primary effector cells in pulmonary fibrosis. Myofibroblasts are characterized by increased production of ECM proteins and by the development of a contractile phenotype with stress fibers that are connected with the ECM via focal adhesions and between cells via adherens junctions. In the liver, myofibroblasts are additionally recruited from hematopoetic stem cells that follow an activation process and from epithelial cells that undergo EMT. The myofibroblast progenitor after injury of different tissues seems to be the locally residing fibroblast, which transiently differentiates into a proto-myofibroblast that is characterized by α-SMA-negative stress fibers. However, the formation of differentiated myofibroblasts from bone marrow-derived circulating fibrocytes is not clear. PDGF: platelet-derived growth factor; TGFβ1: transforming growth factor; 1L: CTGF: connective tissue growth factor; CCL2: CC motif chemokine ligand 2; CXCL12: CXC motif chemokine ligand 12; α-SMA: α-smooth-muscle actin; ECM: extracellular matrix; EDA-FN: extra-domain-A fibronectin.
Figure 15.4
Model for involvement of CD44v6 and Met due to autocrine TGFβ1 signaling in ILD fibroblasts.
Elevated HGF expression at the onset of chronic injury may support a regenerative process, but repetitive lung injury results in overexpression of TGFβ1 and TGFβ1-induced autocrine signaling that induces a sustained expression of CD44v6 and Met. This activates ILD fibroblasts with subsequent increased collagen matrix synthesis. In normal lung fibroblasts, TGFβ1 treatment also activates the Met and CD44v6 receptors. Although HGF interferes with TGFβ1 signaling, because HGF decreases in a reciprocal manner to the increase in TGFβ1 during the progression of chronic injury in ILD fibrosis, TGFβ1-induced CD44v6 and Met can have a crucial role for the sustained fibrogenic activation of ILD fibroblasts (for reference see the Conclusion text).
Figure 15.5
Model of a positive feedback loop between ERK/EGR-1 activation and CD44v6. (From Ghatak S et al. 2016, revised MS submitted to
J Biol Chem
.)
EGR1-induced AP-1 activity stimulates CD44v6 splicing.
Figure 15.6
Proposed model for persistent fibrosis in mice after lung injury. (From Ghatak S et al. 2016, revised MS submitted to
J Biol Chem
.)
NOX4-derived ROS facilitates TGFβ1-mediated fibrosis by inducing differentiation of fibroblasts into myofibroblasts and synthesis of extracellular matrix proteins. Our studies indicate that NOX4 increases HAS2 expression and LMW-HA enhances NOX4/ROS activity, suggesting a positive feedback loop between HA and NOX4 activation. ROS then induces cell invasion through an Akt → MMP pathway. Our study also suggests that in response to lung injury, CD44v6 induces NOX4 through regulation of TGFβ1/Smad3 signaling in myofibroblasts, and NOX4 also regulates CD44v6 production. This suggests that the CD44v6 and HA are upregulated by TGFβ1/Smad3 signaling through a feedback loop requiring the presence of NOX4. These studies show the role of HA interaction with CD44v6 in TGFβ1-induced NOX4 expression, myofibroblast activation, and profibrotic responses.
Chapter 16
Figure 16.1
Year-wise publication scenario (2007–2016) generated from the “Scopus” database, using the keywords (a) photobiomodulation, (b) photobiomodulation – wound healing, (c) polysaccharides – wound healing, and (d) polysaccharides – photobiomodulation – wound healing.
Figure 16.2
Schematic illustration of the beneficial effects of photobiomodulation mediated wound healing.
Figure 16.3
Types of polysaccharides extracted from marine sources intended for wound healing applications.
Chapter 18
Figure 18.1
The wound healing–chronic fibrosis–cancer axis.
Model representing the cellular and molecular mechanisms where if wound healing in acute wound healing settings is not resolved then sustained chronic wound healing helps the feedback loop between chronic fibrosis and cancer progression.
Figure 18.2
Structures of repeating disaccharides of glycosaminoglycans. (Adapted from Misra et al.,
Front Immunol
, 2015.)
Figure 18.3
CD44 protein structures in mice. (Adapted from Misra et al.,
Front Immunol
, 2015.)
Model structure of alternatively spliced CD44 proteins. The CD44 protein is composed of an extracellular N-terminal domain, a stem region in the extracellular domain close to the transmembrane region, where the variant exon products (red/violet circles) are inserted, the transmembrane region, and the carboxyl terminal cytoplasmic tail. There are multiple sites for
N
-glycosylation (purple circles) and
O
-glycosylation (orange circles), and a sulfation domain. The N-terminal portion contains highly conserved disulfide bonds as well as 2 BX7B motifs, both of which are essential for HA binding. CD44 is subjected to extensive glycosylation, sulfation and attachment of GAGs that contribute to regulation of the HA binding activity. The C-terminal cytoplasmic tail contains several phosphorylation sites that regulate the interaction of CD44 with the cytoskeletal linker proteins, as well as with SRC kinases.
Figure 18.4
The involvement of HA and
CD44 in cell survival and differentiation.
(Adapted from Misra et al.,
Front Immunol
, 2015.)
(a) Model for the involvement of CD44v6 and Met due to autocrine TGFβ1 signaling in lung fibrogenic fibroblasts. The repetitive lung injury in pulmonary fibrosis results in overexpression of TGFβ1 and TGFβ1-induced autocrine signaling that induces a sustained expression of CD44v6 and its coreceptor c-Met. This activates fibrogenic lung fibroblasts with subsequent increased collagen matrix synthesis. Therefore, TGFβ1-induced CD44v6 and Met can have a crucial role for the sustained fibrogenic activation of lung fibroblasts. The CD44–phosphorylated ERM complex initiates activation of transforming growth factor-β receptor 1 and 2 (TGFβRI and II) and the downstream SMAD signaling complex, which contribute to fibrosis. (b) Model for involvement of periostin in HA-CD44 mediated cell survival and differentiation. Matricellular protein (periostin (PN)) binding to β1 or β3-integrin activates FAK, which activates downstream MAPK/Erk and PI3K/Akt to regulate cardiac valve cell growth, survival, differentiation into fibroblasts, and matrix organization (maturation). PN binding to β3-integrin also activates Has2 mRNA expression, Has2 phosphorylation and HA synthesis. The interaction of HA with CD44 may, in turn, amplify the downstream effects of PN on heart valve cushion cell differentiation/maturation processes. (c) Cleavage of the extracellular domain is accompanied by the cleavage of the intracellular domain (ICD) by the presenilin–γ-secretase complex. The CD44 ICD acts together with CBP or p300 as a transcription factor and promotes CD44 transcription and extracellular matrix production.
Figure 18.5
Model for involvement of CD44v6 and Met due to autocrine TGFβ1 signaling in ILD fibroblasts. (Adapted from Ghatak et al., Chapter 15 in this book.)
Elevated HGF expression at the onset of chronic injury may support a regenerative process, but repetitive lung injury results in overexpression of TGFβ1 and TGFβ1-induced autocrine signaling that induces a sustained expression of CD44v6 and Met. This activates ILD fibroblasts with subsequent increased collagen matrix synthesis. In normal lung fibroblasts, TGFβ1 treatment also activates the Met and CD44v6 receptors. Although HGF interferes with TGFβ1 signaling, because HGF decreases in a reciprocal manner to the increase in TGFβ1 during the progression of chronic injury in ILD fibrosis, TGFβ1-induced CD44v6 and Met can have a crucial role for the sustained fibrogenic activation of ILD fibroblasts (for reference see the conclusion section).
Figure 18.6
Model of a positive feedback loop between ERK/EGR-1 activation and CD44v6.
EGR1-induced AP-1 activity stimulates CD44v6 splicing.
Figure 18.7
Proposed model for persistent fibrosis in mice after lung injury.
NOX4-derived ROS facilitates TGFβ1-mediated fibrosis by inducing differentiation of fibroblasts into myofibroblasts and synthesis of extracellular matrix proteins. Our studies indicate that NOX4 increases HAS2 expression and low molecular weight (LMW)-HA enhances NOX4/ROS activity, suggesting a positive feedback loop between HA and NOX4 activation. ROS then induces cell invasion through an Akt → MMP pathway. Our study also suggests that in response to lung injury, CD44v6 induces NOX4 through regulation of TGFβ1/Smad3 signaling in myofibroblasts and NOX4 also regulates CD44v6 production. This suggests that the CD44v6 and HA are upregulated by TGFβ1/Smad3 signaling through a feedback loop requiring the presence NOX4. These studies show the role of HA interaction with CD44v6 in TGFβ1-induced NOX4 expression, myofibroblast activation, and profibrotic responses.
Figure 18.8
CD44- and CD44v-induced RTK for apoptosis resistance. (Adapted from Misra et al.,
Front Immunol
, 2015.)
CD44-HA binding, accompanied by activation of CD44-associated Src, ezrin phosphorylation and PI3K activation leads to the lipid-raft-integrated assembly of a complex that includes heat shock protein 70 (HSP70), the cochaperone CDC37, Rho-GEF, Grb2/VAV2, and Gab-1/PI3-kinase (PI3K), which promotes phosphorylation and activation of the receptor tyrosine kinases (RTKs), including ERBB2, ERBB3, EGFR, IGF1R-β, PDGFR-β, and c-MET. CD44-HA binding initiates crosstalk between RTKs, non-RTKs (SRC (Src)), and linker proteins. Studies have indicated that ERBBB2 most likely complexes with CD44 via interactions with Grb2 and Vav2, whereas the interaction of PI3K and CD44 is mediated by Gab-1. PI3K activates Akt and downstream anti-apoptotic events, which contribute to drug resistance. However, HA and PI3K stimulate MDR1 expression, and the stimulatory effects of PI3K would be due mainly to its feedback stimulation of HA production by a positive feedback loop. Studies indicate that MDR1 is associated with CD44 in a lipid microdomain and can be linked via CD44 with the actin cytoskeleton so that expression of both CD44 and MDR1 are concomitantly regulated.
Figure 18.9
CD44- and CD44v-induced COX2/PGE2 for apoptosis resistance. (Adapted from Misra et al.,
Front Immunol
, 2015.)
In colorectal cancer the CD44–ERBB2 complex provides a strong apoptotic resistance through stimulation of cyclooxygenase 2 (COX2) transcription via PI3K and β-catenin. COX2-induced PGE2 stimulates HA synthesis and HA-CD44 signaling. CD44v6 also binds hepatocyte growth factor (HGF) and presents it to c-MET. Activation of MET and the downstream signaling cascades require sustained activation of CD44-associated phosphorylated ezrin, radixin, and moesin (ERM) and SRC (Src) signaling via the Ras-MAPK and the PI3K-Akt pathway. The Ras-Erk pathway can augment CD44v6 synthesis through a feedback loop between the CD44v6 and c-Met/Ras-Erk pathway.
Figure 18.10
Schematic illustration of cellular uptake of plasmid DNA/Tf-PEG-PEI (nanoparticles) polyplexes, their shielding from non-specific interaction, and the mechanism of action of shRNA. (Adapted from Misra et al.,
Front Immunol
. 2015.)
Internalization of PEG-shielded and Transferrin receptor (Tf-R)-targeted polyplexes into target cells occurs by receptor-mediated endocytosis after association of polyplex ligand Tf to Tf-R present on the target cell plasma membrane. Internalized particles are trafficked to endosomes followed by endosomal release of the particles and/or the nucleic acid into cytoplasm. Released siRNA will form an RNA-induced silencing complex and will be guided for cleavage of complementary target mRNA in the cytoplasm. siRNA (antisense) guide strand will direct the targeted RNAs to be cleaved by RNA endonuclease. Finally, plasmid/Tf-PEG-PEI-nanoparticle delivery in the target cell shows reporter gene expression and activity. The normal tissue cells are not affected because they do not make the targeted CD44 variant. Tf-PEG-PEI nanoparticle coated plasmids (pSico-CD44v6shRNA/pFabpl-Cre) circulating in blood accumulate at tumor regions enhanced by the EPR effect. Endocytosis mediated by ligand–receptor interactions occurs because the nanoparticles are coated with the Tf-ligand for the Tf-R receptor on the tumor cell surface.
Guide
Cover
Table of Contents
Chapter
Pages
iii
iv
vii
viii
ix
x
1
2
4
5
6
8
9
10
11
12
13
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
79
80
81
82
83
84
85
86
87
89
90
91
92
93
94
95
96
97
98
99
100
101
103
104
106
107
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
126
127
128
129
130
131
132
133
134
135
136
137
138
139
140
141
142
143
144
145
146
147
149
150
151
152
153
154
155
156
157
158
159
160
161
162
163
164
165
167
168
169
170
171
172
173
174
175
177
178
179
180
181
182
183
184
185
186
187
188
189
190
191
192
193
194
195
196
197
198
199
200
201
202
203
205
206
207
208
209
211
212
213
214
215
216
217
220
221
222
223
225
226
227
228
229
230
231
232
233
234
235
236
237
239
240
241
242
243
244
245
246
247
248
249
250
251
252
253
254
255
256
257
258
259
260
261
262
263
264
265
266
267
268
269
270
271
272
273
274
275
276
277
278
279
e1
List of Contributors
Heidi Abrahamse
PhD
Laser Research Centre University of Johannesburg Johannesburg, South Africa
Daniel D. Bikle
MD, PhD
VA Medical Center and University of California San Francisco, CA, USA
Johanna M. Brandner
PhD
Department of Dermatology and Venerology University Hospital Hamburg-Eppendorf Hamburg, Germany
Olivier Alexandre Branford
PhD
Queen Victoria Hospital East Grinstead, West Sussex, UK
Joanna Bukowska
PhD
Institute of Animal Reproduction and Food Research, Polish Academy of Sciences Olsztyn, Poland
Bruce A. Bunnell
PhD
Center for Stem Cell Research and Regenerative Medicine and Department of Pharmacology Tulane University School of Medicine New Orleans, LA, USA
Melissa Crawford
BSc (Hons)
Department of Physiology and Pharmacology Children's Health Research Institute and Lawson Health Research Institute The University of Western Ontario London, Ontario, Canada
Lina Dagnino
PhD
Department of Physiology and Pharmacology Children's Health Research Institute and Lawson Health Research Institute The University of Western Ontario London, Ontario, Canada
Duncan Hieu M. Dam
PhD
Department of Dermatology Skin Disease Research Center (SDRC) Northwestern University Chicago, IL, USA
Selami Demirci
PhD
National Heart, Lung, and Blood Institute (NHLBI) NIH, Bethesda MD, USA
Luisa A. DiPietro
DDS, PhD
Center for Wound Healing and Tissue Regeneration University of Illinois at Chicago Chicago, IL, USA
Ayşegül Doğan
PhD
National Cancer Institute (NCI) NIH, Frederick MD, USA
Eduardo Escario
MD
Dermatology Service University General Hospital of Albacete Associate Professor, University of Castilla-La Mancha School of Medicine Spain
Trivia Frazier
PhD
LaCell LLC, New Orleans, LA, USA and Center for Stem Cell Research & Regenerative Medicine and Department of Structural and Cellular Biology Tulane University School of Medicine New Orleans, LA, USA
Barbara Gawronska-Kozak
DSc, PhD
Institute of Animal Reproduction and Food Research, Polish Academy of Sciences Olsztyn, Poland
Shibnath Ghatak
PhD
Department of Regenerative Medicine and Cell Biology Medical University of South Carolina Charleston, SC, USA
Jeffrey M. Gimble
MD, PhD
LaCell LLC, New Orleans LA, USA and Center for Stem Cell Research and Regenerative Medicine and Departments of Structural and Cellular Biology, Medicine, and Surgery Tulane University School of Medicine New Orleans, LA, USA
Vincent C. Hascall
PhD
Department of Biomedical Engineering Cleveland Clinic Cleveland, OH, USA
Nicolette Nadene Houreld
DTech
Laser Research Centre University of Johannesburg Johannesburg, South Africa
Ander Izeta
PhD
Tissue Engineering Laboratory, Bioengineering Area Instituto Biodonostia, Hospital Universitario Donostia and Department of Biomedical Engineering, School of Engineering Tecnun-University of Navarra San Sebastián, Spain
Sophia A. Jelsma
BS
Department of Dermatology Skin Disease Research Center (SDRC) Northwestern University Chicago, IL, USA
Francisco Jimenez
MD
Mediteknia Dermatology and Hair Transplant Clinic Associate Professor University Fernando Pessoa Canarias, Gran Canaria, and Medical Pathology Group, ULPGC Canary Islands, Spain
Jasreen Kular
BSc, BMedSci(Hons), PhD
Centre for Cancer Biology SA Pathology and University of South Australia Adelaide, South Australia, Australia
Sathish Sundar Dhilip Kumar
PhD
Laser Research Centre University of Johannesburg Johannesburg, South Africa
Feyzan Őzdal Kurt
PhD
Department of Biology Faculty of Sciences and Letters Manisa Celal Bayar University Manisa, Turkey
Jeffery T. Kwock
MD
Department of Dermatology and Immunology Duke University Medical Center Durham, NC, USA
Alexandra Laberge
MD, Msc
Centre de Recherche en Organogénèse Expérimentale de l'Université Laval/LOEX Department of Surgery, Faculty of Medicine Université Laval and Centre de Recherche du CHU de Québec-Université Laval Québec, QC, Canada
Michael T. Longaker
MD, MBA
Department of Surgery and Reconstructive Surgery The Stanford University Medical Center Stanford, CA, USA
H. Peter Lorenz
MD
Department of Surgery and Reconstructive Surgery The Stanford University Medical Center Stanford, CA, USA
Amanda S. MacLeod
MD
Department of Dermatology and Immunology, Duke University Medical Center Durham, NC, USA
Roger R. Markwald
PhD
Department of Regenerative Medicine and Cell Biology Medical University of South Carolina Charleston, SC, USA
Clement D. Marshall
MD
Department of Surgery and Reconstructive Surgery The Stanford University Medical Center Stanford, CA, USA
María Luisa Martínez
MD
Dermatology Service Hospital General Universitario of Albacete Spain
Suniti Misra
PhD
Department of Regenerative Medicine and Cell Biology Medical University of South Carolina Charleston, SC, USA
Alessandra A. Moore
MD
Department of Surgery and Reconstructive Surgery The Stanford University Medical Center Stanford, CA, USA
Ricardo Moreno Rodriguez
PhD
Department of Regenerative Medicine and Cell Biology Medical University of South Carolina Charleston, SC, USA
Véronique J. Moulin
PhD
Centre de Recherche en Organogénèse Expérimentale de l'Université Laval/LOEX Department of Surgery, Faculty of Medicine Université Laval and Centre de Recherche du CHU de Québec-Université Laval Québec, QC, Canada
Paul E. O'Brien
MD
Hematology/Oncology Division Medical University of South Carolina Charleston, SC, USA
Yuko Oda
PhD
VA Medical Center and University of California San Francisco, CA, USA
Amy S. Paller
MD
Department of Dermatology, Skin Disease Research Center (SDRC) Northwestern University Chicago, IL, USA
Hayley S. Ramshaw
BSc (Hons), PhD
Centre for Cancer Biology SA Pathology and University of South Australia Adelaide, South Australia, Australia and Adelaide Medical School Faculty of Health and Medical Sciences University of Adelaide Adelaide, South Australia, Australia
Kerstin J. Rolfe
PhD
BCOM London, UK
Fikrettin Şahin
PhD
Department of Genetics and BioEngineering Faculty of Engineering Yeditepe University Kayisdagi, Istanbul, Turkey
Michael S. Samuel
BSc (Hons), PhD
Centre for Cancer Biology SA Pathology and University of South Australia Adelaide, South Australia, Australia and Adelaide Medical School Faculty of Health and Medical Sciences University of Adelaide Adelaide, South Australia, Australia
Megan Schrementi
PhD
Department of Science and Health, DePaul University Chicago, IL, USA
Amy Lin Strong
MD PhD
Center for Stem Cell Research and Regenerative Medicine and Departments of Structural and Cellular Biology Tulane University School of Medicine New Orleans, LA, USA
Chia-ling Tu
PhD
VA Medical Center and University of California San Francisco, CA, USA
Elgin Türköz Uluer
MD
Department of Histology and Embryology Faculty of Medicine Manisa Celal Bayar University Manisa, Turkey
Hafize Seda Vatansever
MD, PhD
Department of Histology and Embryology Faculty of Medicine Manisa Celal Bayar University, Manisa, Turkey and Experimental Health Research Center of Health Sciences Near East University Mersin, Turkey
Thomas Volksdorf
PhD
Department of Dermatology and Venerology University Hospital Hamburg-Eppendorf Hamburg, Germany
Xiying Wu
MD
LaCell LLC New Orleans, LA, USA
Chapter 1Stem Cell Regeneration and Repair – Overview
Clement D. Marshall, Alessandra A. Moore, Michael T. Longaker and H. Peter Lorenz
Department of Surgery and Reconstructive Surgery, The Stanford University Medical Center, Stanford, CA, USA
Introduction
While at first glance the skin appears to be no more than a static unchanging surface, it is in fact a complex, dynamic organ that continuously replenishes its cellular and molecular content. In addition to this homeostatic maintenance, skin has evolved the remarkable ability to rapidly repair itself after injury. For our most distant ancestors, there was presumably evolutionary pressure to rapidly restore the barrier function of skin before infection could set in. The result of this evolutionary necessity is scar tissue, which serves as a protective barrier, but falls short in several ways compared with uninjured skin. In humans, the end point of healing all but the smallest injuries is the formation of scar [1]. For most people, scars are a cosmetic concern, but many patients are affected by major scars that result in debilitating contractures as well as disfigurement in aesthetically sensitive areas such as the face. Children with major scars in visible areas such as the face often suffer from long-term psychological stress and impaired self-esteem [2]. If excessive scar formation represents one end of the human wound healing spectrum, the other end consists of chronic and non-healing wounds. Often arising in patients with diabetes, peripheral arterial disease, impaired mobility, and other comorbidities, chronic wounds typically require months of intensive treatment and consume substantial healthcare resources [3].
Stem cells are a key cellular player in the repair of skin after injury and during normal homeostasis. Tremendous progress has been made in recent years toward delineating the role of stem cells in these processes. An improved understanding of the identities of skin stem cells as well as the signals that govern their behavior will hopefully allow for the development of improved therapies for scarring and poorly healing wounds. This chapter will begin with an overview of the events of normal wound healing and stem cell biology and will then review our current understanding of the role of stem cells in skin regeneration and repair.
Overview of Skin Wound Healing
The major function of skin is to provide a barrier that excludes noxious and infectious agents of the outside world while protecting underlying structures from trauma and preventing the loss of valuable body fluid. Wound repair appears to have evolved in a way that rapidly restores these functions while simultaneously preventing infection of the wound (Figure 1.1).
Figure 1.1There are three classic stages of wound repair: (a) inflammation, (b) new tissue formation, and (c) remodeling.(a) Inflammation. This stage lasts until about 48 h after injury. Depicted is a skin wound at about 24–48 h after injury. The wound is characterized by a hypoxic (ischaemic) environment in which a fibrin clot has formed. Bacteria, neutrophils, and platelets are abundant in the wound. Normal skin appendages (such as hair follicles and sweat duct glands) are still present in the skin outside the wound. (b) New tissue formation. This stage occurs about 2–10 days after injury. Depicted is a skin wound at about 5–10 days after injury. An eschar (scab) has formed on the surface of the wound. Most cells from the previous stage of repair have migrated from the wound, and new blood vessels now populate the area. The migration of epithelial cells can be observed under the eschar. (c) Remodeling. This stage lasts for a year or longer. Depicted is a skin wound about 1–12 months after repair. Disorganized collagen has been laid down by fibroblasts that have migrated into the wound. The wound has contracted near its surface and the widest portion is now the deepest. The re-epithelialized wound is slightly higher than the surrounding surface and the healed region does not contain normal skin appendages. (Reproduced from Gurtner et al. [1], with permission from Nature Publishing Group.)
The first events after a skin injury has occurred relate to the restoration of hemostasis. A fibrin and platelet plug prevents ongoing bleeding from blood vessels. The fibrin matrix that composes the plug provides a scaffold for wound healing cells that will migrate in later. Activated platelets in the injury provide early chemical signals that activate other cells and potentiate further wound healing events [4].
The first phase of a true wound repair is known as the inflammatory phase. Immune cells such as macrophages, neutrophils, and lymphocytes enter the wound tissue and begin the process of removing bacteria, dead cells, and other debris [5]. Cytokines released during wounding and hemostasis are critical for the recruitment of these immune cells to the wound [4]. Immune cell influx is accompanied by a local inflammatory reaction characterized by increased blood flow and capillary leaking, causing the typical symptoms of redness, swelling, and increased warmth. In addition to cleaning the wound area and removing infectious agents, immune cells release a host of cytokines and other chemical mediators that encourage other cells to engage in healing behaviors [4].
Inflammation is followed by the proliferative phase of wound healing. This refers to the migration of cells into the wound, particularly fibroblasts and keratinocytes, that are responsible for building new tissue to reconstruct the wound. These cells are highly responsive to chemical mediators released by immune cells during the inflammatory phase [6]. New epidermis and dermis are constructed to replace the empty space left by the wound during this phase. In almost all wounds, the new skin is built in the form of scar [1]. Compared with normal skin, scar lacks hair follicles and sweat glands, is stiffer, and is often raised and hyperpigmented. The basement membrane of the epidermis in scar is flat and does not contain the rete pegs that normally project down into the dermis [7] (Figure 1.2). Large scars, particularly those located over a joint, often contract as a result of myofibroblast action. This contraction occurs in the remodeling phase of wound healing, during which scar extracellular matrix, including collagen, is extensively remodeled [4]. Contractures can be painful and cause severe physical impairment, particularly in patients with large burn scars [8].
Figure 1.2Masson's trichrome staining of the interface between normal (left) and scarred (right) dorsal skin in the adult mouse.Normal skin contains hair follicles and other dermal appendages. Scarred skin does not contain these appendages and the epidermis is flattened. Note that that scarred dermis is thicker than the normal dermis. Scale bar, 500 μm.
While stem cells are normally active at a low level in uninjured skin to maintain homeostasis, they are recruited during the proliferative phase of wound healing to provide large numbers of new cells to populate the healing wound [9].
Stem Cell Definition: History
Most cells have short life spans and are not capable of indefinite self-renewal. In the 1970s, the concept of the stem cell was developed to describe a special population of cells that divide in order to replenish a population of differentiated cells but do not themselves differentiate. Over time the definition of the stem cell has evolved [10]. Today, stem cells are generally considered to be undifferentiated cells that self-renew and that produce differentiated cells as progeny [11]. Within this broad definition there are many types of stem cells that differ based on their capacity for long- or short-term self-renewal and the number of different cell types that they produce [11].
The presence of stem cells has been verified in most tissues of the body, although certain tissues such as the pancreas may not contain stem cells [10, 12]. The precise manner in which a stem cell behaves and produces differentiated progeny differs depending on the tissue involved. Cell surface markers and genes expressed by stem cells also differ markedly between tissues, making identification and isolation of stem cells sources challenging. Many stem cells express regulatory genes that are switched off in their progeny, making the identification of the progeny cells in vivo more difficult. Furthermore, a stem cell's progeny may change its morphology, cell surface marker expression, or migrate to a new location.
A lineage can be defined as a particular population of cells and all subsequent cells descended from them, regardless of location or phenotype. The concept of lineage tracing was developed in order to follow cell offspring as they migrate to new locations and change surface marker and gene expression profiles [13] (Figure 1.3). Lineage tracing comprises multiple techniques that involve physically or genetically marking cells or populations of cells with a reporter in such a way that all of their progeny retain the reporter. This allows cells of a certain lineage to be detected even if they change location, gene expression, or surface marker profile [22].
Figure 1.3Different approaches to lineage tracing.(a) Direct observation, as pioneered by Whitman and colleagues (exemplified by a plate from Conklin, 1905) [14]. (b) Schematic showing agar chips with vital dyes applied on to the surface of an early stage amphibian embryo (top). These dyes label regions within later stage embryos (bottom) (based on Vogt, 1929; adapted from Gilbert, 2000 [15, 16]). (c) Use of soluble carbocyanine dyes to fate map chick neural crest. (Reproduced from Serbedzija et al., 1989, with permission from The Company of Biologists Ltd [17].) (d) Whole-mount of mouse epidermis showing DNA label-retaining stem cells in the hair follicle bulge. (Reproduced from Braun et al., 2003 [18].) Red: keratin 14; green: BrdU. Scale bar: 100 μm. (e) LacZ retroviral vector introduced into rat retinal cells (upper panel) and subsequently tracked in the reconstituted retina. (Reproduced from Price et al., 1987, with permission from C. Cepko [19].) (f) Schematic showing Spemann and Mangold's organizer experiment, which was performed by grafting tissues between amphibian embryos (adapted from Grove, 2008 [20]). (g and h) Adult mouse chimeras from GFP-positive and -negative mice. (g) Whole-mount of lung (reproduced from Giangreco et al., 2009 [21]). (h) Histology of skin tumor (reproduced from Arwert et al., 2010 [22]). The GFP-positive region in (h) is brown. (Reproduced with permission from Kretzschmar and Watt [13].)
In perhaps the most powerful and adaptable method for lineage tracing, a gene expressing the Cre recombinase enzyme is inserted into the genome under the control of a specific gene promoter that defines a lineage (i.e., the gene of interest). A reporter gene is inserted separately. When the gene of interest is expressed, the Cre recombinase permanently activates the reporter gene by altering the reporter gene's genetic sequence in that cell. In this way, the reporter remains activated in the cell and in all of its offspring, but not in cells that never expressed the gene of interest [24]. Further refinements of this technique allow the activation of the reporter gene to be inducible. In this way, a researcher can choose a specific time point to begin the lineage tracing. Lineage tracing with inducible methods has allowed for more precise characterization of the behavior of cells at different time points in the developing embryo, which in turn has revolutionized the field of developmental biology [25].
Stem Cells in Skin Homeostasis and Repair
Lineage tracing using Cre recombinase has been critical in defining the identities and roles of various stem cell populations of the skin. These experiments are generally carried out in mice and, as a result, much of our knowledge of skin stem cell biology may not be totally applicable to other species. In general, the stem cells of a particular structure within the skin are mainly responsible for maintaining the cell population of that niche. Often, though, stem cells based in a given location give rise to progeny that migrate to other locations and participate in the regeneration of distant tissues.
The skin contains several structures that each contain their own unique compartment of stem cells. These include the interfollicular dermis, hair follicles, sebaceous glands, eccrine sweat glands, and dermal papillae. Another group of stem cells that participate in skin regeneration and repair are circulating mesenchymal stem cells.
Interfollicular Epidermis
The interfollicular epidermis (IFE) is the region of epidermis located between hair follicles. In the uninjured state, stem cells residing in the basal layer of the IFE divide at a steady rate in order to provide new keratinocytes to populate the epidermis [9]. These cells are characterized by expression of the gene Lrig1 [26]. Loss of Lrig1 in these cells results in hyperproliferation of epidermal cells, suggesting that it has a role in preventing excessive growth. Lrig1+ cells also contribute to the growth of sebaceous glands and hair follicles [26].
The gene Lgr6 is a marker of primitive epidermal stem cells that in the prenatal state establish all lineages of the skin, including cells of the hair follicle, sebaceous gland, and interfollicular dermis. In postnatal life, Lgr6-expressing cells reside above the hair follicle bulge and regenerate the sebaceous gland and the IFE [27].
While the IFE can receive contributions of cells from other structures such as the hair follicle, the IFE is also capable of repairing and renewing itself in the absence of these other cells [9].
Hair Follicle
The cellular content of the hair follicle is exceptionally well studied and serves as a model for stem cell biology as a whole. The hair follicle is a complex structure with several distinct regions that contain unique populations of hair follicle stem cells (HFSCs) (Figure 1.4). While the HFSCs can regenerate the follicle itself, an important unresolved question is to what extent HFSCs have a meaningful role in regeneration of the skin following injury.
Figure 1.4Heterogeneity of epidermal and hair follicle stem cells.Skin epithelia feature distinct stem cell populations both in epidermis and, most prominently, in hair follicles. Epithelial stem cells in different microanatomical locations have different lineage potentials. Stem cells in the follicular infundibulum and interfollicular epidermis physiologically are restricted to epidermal fate. Interfollicular epidermal stem cells can be identified as slow-cycling in label retention studies, but distinct markers remain elusive. The isthmus and junctional zone of hair follicles harbor several distinct epithelial cell populations. Most prominent among them are Lrig1+ (yellow), Gli1+, and Lgr6+ stem cells (green), all of which physiologically maintain the isthmus and contribute to sebaceous gland, infundibulum and in some instances to interfollicular epidermis. Blimp1 identifies unipotent sebaceous gland progenitors (orange). The bulge stem cells (blue) normally contribute to all hair follicle lineages and can be identified based on the expression of Krt15, CD200, Lgr5, CD34, Sox9, Lhx2, Tcf3 and Nfatc1. The secondary germ of telogen hair follicles (purple) contains committed hair follicle-fated progenitors that express CD200, Gli1 and Lgr5. (Reproduced from Plikus et al. [9], with permission from Elsevier.)
In the developing embryo, the hair follicle is initially formed by separate populations of HFSCs expressing Lhx2 and Sox9, respectively [28]. The Lhx2+ cells appear to contribute transiently to hair follicle development while Sox9+ cells persist for longer. The developed hair follicle normally cycles through three stages of growth: catagen (regression), telogen (resting), and anagen (growth), a process that also involves varying contributions from different HFSC populations [28].
Stem cells residing in the bulge region of the hair follicle were the first HFSCs to be discovered and are characterized by expression of the genes Krt15, Lgr5, and Gli1, among others [9, 29]. Initially it was thought that these cells could contribute to skin regeneration, since they were found in the epidermis after a scratch injury [30]. However, subsequent sophisticated analyses revealed that while bulge cells transiently contribute progeny to the healing epidermis, these cells eventually disappear and in the long-term bulge stem cells are only capable of regenerating the hair follicle itself [9].
The junctional zone of the hair follicle is above the bulge and adjacent to the sebaceous gland. It contains a complex population of stem cells that are generally defined by expression Lrig1 but express other markers differently and have different roles in regeneration [9, 26]. Those expressing Lgr6 in prenatal life contribute to the formation of the hair follicle, sebaceous gland, and interfollicular dermis. Postnatally, Lgr6+ cells contribute to repair of IFE and hair follicles. Given that these cells are capable of forming several skin structures, it has been proposed that they are the most primitive skin stem cell [27].
Sebaceous Gland
The sebaceous gland is a separate structure that is intimately connected with the hair follicle as it secretes sebum on to the hair shaft. A population of unipotent stem cells defined by expression of Blimp1 is thought to control cellular contribution to the sebaceous gland [31]. Interestingly, ablation of Blimp1 does not cause loss of the sebaceous gland but rather hyperplasia, suggesting that Blimp1 serves an inhibitory role in sebaceous gland maintenance. Recently evidence has emerged that Blimp1+ cells of the sebaceous gland may in fact represent terminally differentiated cells rather than true stem cells [32]. While they are required for homeostatic maintenance of the sebaceous gland, they do not appear to produce progeny that replenish the cellular population of the gland.
Sweat Gland
The eccrine sweat gland is separate from the hair follicle and is responsible for secretion of sweat on to the skin surface. The nature of sweat gland cells remains less well understood than that of the hair follicle. Lu et al. demonstrated that the sweat gland itself and the duct that connects the gland to the skin contain separate populations of epithelial cells. Duct





























